BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1471-en.html


 , Mohammad Yousef Alikhani2
, Mohammad Yousef Alikhani2 
 , Willem B. Van Leeuwen3
, Willem B. Van Leeuwen3 
 , Ali Mojtahedi4
, Ali Mojtahedi4 

 , Sima Kazemi5
, Sima Kazemi5 

 , Pezhman Karami6
, Pezhman Karami6 


2- Brucellosis Research Center, Hamadan University of Medical Sciences, Hamadan, Iran ,
3- University of Applied Sciences Leiden, Zernikedreef 11, Leiden, The Netherlands
4- Department of Microbiology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
5- Brucellosis Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
6- Department of Microbiology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Burn and Antimicrobial Resistant Pathogens
Burn injuries are still one of the main causes of death in the US. About 486,000 burn injuries are annually admitted to the emergency department (2). More than 60% of the US acute cases of hospi-talization are related to burn injuries and were admitted to 128 burn centers (2, 3).
The immune response of burn injuries is instant, fulminant, and severe. Immunosuppression may help patients to survive (4). Extensive and deep thermal injuries reduce both the cellular as well as the humoral immune defense systems. After the thermal injury, reduction in lymphocyte proliferation and conseq-uently mixed lymphocyte response are triggered by the release of prostaglandins, kinins, superoxides, leukotrienes, and anaphylatoxins. The formation of membrane attack complexes (MAC), decrease in immunoglobulin levels, and activation of complement lead to cytolysis (5).
Immediate antibiotic treatment of a burn wound infection is highly relevant. It will improve wound healing, limit the formation of scarring, and prevent bacteremia, sepsis, or multiple-organ dysfunction syndrome (1). The main cause of death (28%) in burn patients is sepsis. Additional to antibiotic therapy, topical burn wound medication (see Table 1) reduces the risk of burn wound sepsis (6).
Bacteria is the major cause of burn ulcers. These microbes may form biofilms in burn wounds within 48-72 hours after injury [1]. These organisms are commensal skin flora, the intestinal or the respiratory tract flora of the patient. These organisms can also originate from contact with contaminated environ-ments or (hands of) co-workers (7). The most common pathogens are listed in Table 2.
Table 1. Commonly used antimicrobial agents
| Agent Class | Description | Application | Ref |
|---|---|---|---|
| Topical antibiotics | (8-21) |
||
| Mafenide acetate | Sulfamylon acetate cream is a broad-spectrum antibiotic that affects Gram-negative bacteria, especially Pseudomonas aeruginosa, but little activity against aerobic Gram-positive bacteria | Clinical 2nd/3rd-degree burns | |
| Bacitracin | This is a good alternative for silver sulfadiazine in burn patients with allergies to sulfa | Clinical 2nd/3rd-degree burns | |
| Mupirocin | An inhibitor of Gram-positive skin flora such as Staphylococcus aureus and coagulase-negative staphylococci | Clinical 2nd/3rd-degree burns | |
| Neosporin | An ointment containing bacitracin, neomycin, and polymyxin B | Clinical 2nd/3rd-degree burns | |
| Nitrofurazone | Nitrofurazone is a disinfectant against both gram-negative and gram-positive bacteria | Clinical 2nd/3rd-degree burns | |
| TOPICAL SILVER PREPARATIONS | |||
| Silver nitrate | Silver nitrate is usually given topically by gauzes in patients with severe burns. Some references show that nitrate is toxic to tissues and wounds |
Clinical 2nd/3rd-degree burns | |
| Silver sulfadiazine (SSD) | SSD is a gold standard in burn treatment |
Clinical 2nd/3rd-degree burns | |
| Cerium nitrate-SSD | Burnt skin makes a lipid-protein complex that suppresses the immune system. Cerium nitrate denatures this lipid complex protein, thus preventing suppression of the immune system | Clinical 2nd/3rd-degree burns | |
| Sustained silver Releasing systems | Silver Nitrate, SSD, and Cerium Nitrate-SSD are silver products in solutions, salts, or compounds used for gauze spraying. Silver-based dressings are newer products that are used alone | Clinical 2nd/3rd-degree burns | |
| Silver-impregnated biological material | Silver incorporated into the amniotic membrane is more effective than the amniotic membrane alone | Clinical 2nd/3rd-degree burns | |
| IODINE PREPARATIONS | |||
| Povidone-Iodine | The povidone-iodine solution is active against a wide spectrum of bacteria, fungi, protozoa, and viruses | Clinical 2nd/3rd-degree burns |
|
| Cadexomer iodine | Cadexomer iodine is an antimicrobial product. There are some reports that show cadexomer iodine has effectiveness against S. aureus and MRSA |
Clinical chronic wounds |
|
| PHOTODYNAMIC THERAPY | Light with the wavelength excites the PS (photosensitizer) to its exciting uniqueness, which can pass through the system into the exciting triple mode for a long time. In the presence of oxygen, the triple state of PS produces energy into the molecular oxygen of the ground state (a triplet), which produces reactive oxygen species (ROS) and can kill microbial cells |
||
| CHITOSAN PREPARATIONS | Chitosan has antimicrobial effects due to destruction of the outer membrane and permeabilization of the plasma membrane |
Clinical 2nd-degree burns | |
| ANTIMICROBIAL PEPTIDES | Antimicrobial peptides are depicted to kill gram-positive bacteria and gram-negative (especially strains that are resistant to routine antibiotics), Mycobacteria (including Mycobacterium tuberculosis). The antimicrobial peptides also have the ability to improve immunity (22) |
Table 2. Pathogens responsible for burn infections and their occurrence in drug resistance
| Group | Species | Drug Resistance |
|---|---|---|
| Gram-positive bacteria | S. aureus | |
| Methicillin-resistant S. aureus | By definition | |
| Coagulase-negative staphylococci | MRSE (methicillin-resistant Staphylococcus epidermidis) increasing |
|
| Enterococcus sp. | ||
| Vancomycin-resistant enterococci | by definition | |
| Gram-negative bacteria | P. aeruginosa | High innate resistance |
| Escherichia coli | ESBL(extended spectrum beta-lactamases) increasing | |
| Klebsiella pneumoniae | ESBL increasing | |
| Serratia marcescens | increasing | |
| Enterobacter sp. | ESBL increasing | |
| Proteus sp. | ESBL increasing | |
| Acinetobacter sp. | High innate resistance | |
| Bacteroides sp. | uncommon |
Overuse and misuse, wrong prescription, extensive veterinary and agricultural use, lack of rapid laboratory tests, poor hygiene, sanitation practices, poor infection control measurements in healthcare, lack of new antibiotics are the reasons for the worldwide emergence of antibiotic resistance (24, 28).
Main Body
Treatment of Burn Wound Infections
Phage therapy
Bacteriophages (phages) were found by Frederick Twort in 1915 and Félix d'Herelle in 1917, eleven years before penicillin (1928) was discovered. The name was formulated from "bacteria" and "phagein" (Greek: to eat or devour). It is estimated that phages in the biosphere are about 1031, showing their extreme abundance [32] (30).
General Characteristics of Bacteriophages
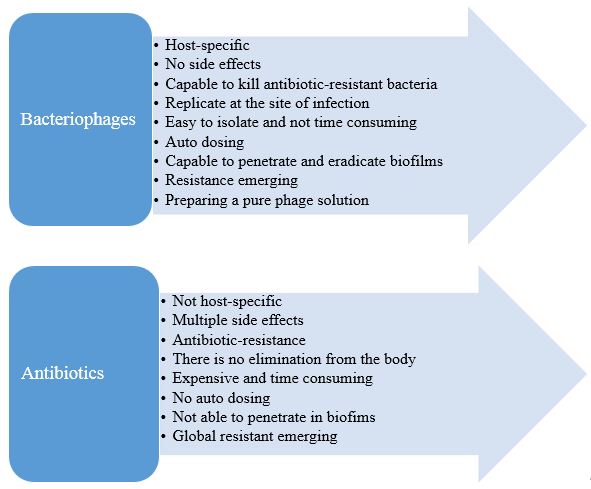
Table 3. Advantages of phages over antibiotics
| Bacteriophages | Antibiotics |
|---|---|
| Specific, do not affect the commensal flora (33, 34) | Antibiotics affect both pathogenic and natural microorganisms. This may lead to patient's microbial unbalance, which may cause secondary infections (34) and antibiotic resistance |
| No descriptions of any serious side effects | Multiple side effects (35) |
| Phages capable to kill antibiotic-resistant bacteria (36) | Antibiotic-resistance is considerable these days |
| Phages replicate and are available at the site of infection | They are eliminated from the body and do not necessarily exist at the site of infection |
| Selection of new phages is a process that takes only several days or weeks |
Developing a new antibiotic is a time-consuming process that can take several years |
| Phages are self-replicative and easy to isolate (35) | Antibiotics are not |
| Other advantages include auto "dosing". Phages during the process of killing bacteria can increase the number, especially in places where the hosts are placed; the phages themselves help to create a dose of phage (37) | - |
| Bacteria in biofilms are more resistant to antibiotics compared with planktonic bacteria. However, phages can clear some biofilms because of the ability for active penetration into the biofilm path by slipping a bacterial layer at once or because of the release of biofilm exopolymer-degrading depolymerizes (21) | - |
| Other advantages of phages are the ability to replicate in situ when a sufficient bacterial population exists; they can reduce phage doses needed to achieve efficacy. Low dose phage can also increase the safety of the product because phages only increase in density if they actively kill bacteria (38) | It is sometimes inevitable to use antibiotics in high dose |
Figure 1. Summary of bacteriophage and antibiotic pros and cons
Antibiotics are relatively indiscriminative and can be applied for patient treatment before the availability of diagnostic microbiological results (31).
There is evidence that shows the anti-inflammatory and immunomodulatory ability of phages (41).
A great concern of phage therapy is the gap between its safety and effectiveness because there is a lack of knowledge in this field. For example, phages can introduce resistance markers in pathogens or commensals. Of concern, bacteria may develop phage resistance after phage therapy; however, a combina-tion of phage and antibiotic therapy could be an interesting option to tackle the phage resistance issue of bacteria (39, 42).
Abedon et al. have listed some key factors that should be considered in phage therapeutical studies, such as the characteristics of phages, target individ-uals (humans) and bacterial dosing, formulations, and phages efficacy. The specificity of most phages in mono-microbial infectious diseases is very eligible, but not in poly-microbial infections, unless the phage is combined with a suitable antibiotic (40).
Phage Therapy Revitalization
Human phage therapy has been used in the early years to treat conditions such as typhoid fever, surgical wound infections, dysentery, peritonitis, septicemia, external otitis media, and urinary tract infections (44). There is a lot of criticism on these studies, and it was aimed at the lack of good method-ological design, standards, controls, characterization of phage preparation, and contradictory results.
Worldwide, some clinical trials have confirmed the importance of phage application in defeating bacteria, for instance: 1. Topical administration (45), Wright et al. tested the effect of a phage cocktail in the treatment of chronic otitis media due to an antibiotic-resistant P. aeruginosa in a double-blind placebo-controlled study. The phage cocktail improved all outcomes compared to the placebo; 2. FagoBurn, a European clinical trial (2015-2017) describing the prophylactic application of phages to prevent skin infections in burn patients, is highly interesting. Full-scale clinical trials for phage therapy are still rare, but a few case studies are published mentioning the phage therapy application (46-48).
Clinical trials were conducted to apply phage therapy to treat or prevent bacterial infections, tuberculosis, and MRSA infections included. Phage therapy has not been approved by the FDA. However, phages have already been applied in experimental therapies in phage therapy centers. The Hirszfeld Institute (Poland) provides phage therapy for various pathogenic genera such as the Enterobacteriaceae family, Acinetobacter, Pseudomonas, Staphylococcus, and Stenotrophomonas. The therapeutic results were positive in 50% of the cases (38, 40). The Eliava Phage Therapy Center (Georgia) provides phages for treating Enterococcus faecalis, various genera of Enterobac-teriaceae, P. aeruginosa, Salmonella, S. aureus, and various genera of the Streptococcaceae family. Some reports described the recovery of severe comatose patients after phage therapy (38).
A mixture of multiple phages (phage cocktail) containing several bacteriophage strains may be useful to increase the antimicrobial activity and may reduce the risk of phage resistance, which is inevit-able. A variety of phage companies that offer phage-based products commercially are listed in Table 4.
Table 4. Phage Companies
| Company | Use of Phages |
|---|---|
| Center for Innovative Phage Applications and Therapeutics (US ) Eliava Phage Therapy Center (GE ) Phage Therapy Center (GE ) Phage Therapy Unit (PL) |
They facilitate patient phage therapy treatment |
| Ecolyse (US ) Fixed Phage (UK ) InnoPhage (PT ) ACDPharma (NO ) |
They provide Phage-mediated biocontrol |
| Biochimpharm (GE ) Imbio (RU ) Microgen (RU ) |
They are involved primarily in phage product distribution |
| Adaptive Phage Therapeutics (US ) AmpliPhi Biosciences (US ) Evolution Biotechnologies (UK ) InnoPhage (PT ) iNtODEWorld (KR ) Phagelux (CN ) Phagomed (AT ) Pherecydes Pharma (FR ) |
They are currently in pre-clinical phage therapy research and development (R&D) step |
| GangaGen (US/IN ) Lysando GmbH (DE ) Micreos Food Safety (NL ) New Horizons Diagnostics? (US ) |
Development of enzybiotics |
| GeneWeave (US ) Micromensio (CA ) Sample6 (US ) |
Phage-based bacterial detection technologies |
| JAFRAL (SI ) Clean Cells (FR ) Paragon Bioservices (US ) |
Phage manufacturing/production for others (49)] |
Phage Therapy of Burn Wound Infections and Biofilm
Animal Model Studies
Another study, using a burnt mice infection model, showed that phage Kpn5 was more effective against K. pneumoniae B5055 than silver nitrate or gentamicin. Furthermore, a phage cocktail in a burn wound infection caused by K. pneumoniae B5055 showed high protection in patients who did not respond to routine antibiotic therapy (52). In contrast, another study described that phages specific to the Podoviridae family were ineffective in controlling P. aeruginosa in infected burnt mice (53). Soltan Dallal et al.'s study in mice suggests that phage SE20 is a promising candidate for controlling salmonellosis caused by Salmonella Enteritis [55].
Sometimes, a phage cocktail has no efficacy to pathogens such as P. aeruginosa. When the bacterial load is hardly reduced by the phage cocktail, bacteria seem to adapt themselves in order to defeat stressors (emerging phage resistance). Here are some possible bacterial resistance mechanisms: 1. Prevention of phage adsorption by loss of modification of bacterial receptors and prevention of phage DNA entry; 2. Degradation of phage DNA by restriction-modification and other related systems (BREX (bacteriophage exclusion), DISARM (defense island system associated with restriction-modification), CRISP-Cas (clustered regularly interspaced short palindromic repeats)); 3. Use of abortive infection systems that block phage replication, transcription, or translation; 4. Cyclic oligonucleotide-based antiphage signaling systems [56]. It is of great importance to study the mechanism of phage resistance in bacteria to prevent phage cocktail resistance of the pathogens. Implementation of phages with a broad host range, targeting various distinct bacterial receptors, may reduce the development of phage resistance (54).
Phage-antibiotic synergy has good results because it increases fitness costs. In a study, the authors described that a mixture of a single antibiotic such as an aminoglycoside (gentamicin) or ciprofloxacin com-bined with two different phages specific to the Myoviridae family have high efficacy against P. aeruginosa infections and can reduce the bacterial inoculum in approximately 2 logs (42). A study showed no reduction in the P. aeruginosa count at culture tube in a combination of phage and antibiotic therapy (55). In developing countries, phage therapy for treating infectious diseases such as cholera can be helpful by designing well-established trials (56).
Biofilm formation is a mechanism produced by bacteria such as P. aeruginosa and S. aureus to be a winner in a race with unfavorable circumstances. As antibiotics cannot penetrate the biofilm, phages are probable candidates to penetrate biofilms. Reports show that a mixture of phages has a remarkable positive effect on the degradation of S. aureus biofilms (57). Phage therapy is also recommended as an effective antimicrobial method to degrade P. aeruginosa biofilm (57, 58). Chegini et al. demons-trated that a mixture of phages with anti-biofilm compounds, such as nanoparticles or enzymes, was more effective than monotherapy of phages. Phages can induce penetration of antibiotics in the internal layer of biofilm by making defects in the extracellular matrix; they can also suppress biofilm formation by hurdling the quorum-sensing (59). Another study mentioned the Trojan horse effect of phages by the eradication of biofilms that are established by both P. aeruginosa and methicillin-resistant S. aureus (60). Phage therapy also effectively prevents E. faecalis biofilm formation (36). Another report declared that bacteriophages act as an alternative bacterial biofilm inhibitor (61). These mentioned reports all showed that phage therapy is a new alternative method in combination with antimicrobial treatment, especially in infections caused by biofilm-producing Gram-negative bacteria. More research is needed for the worldwide introduction of phage therapy to combat infectious diseases.
Phage therapy can be a suitable alternative to defeating (antibiotic-resistant) pathogens in infectious diseases. Burn wound infections need a topical treatment. Phages can be used as a solution for infections with antimicrobial-resistant pathogenic bacteria as a monotherapy or in combination with antibiotic therapy. Numerous studies have demons-trated the potency of phages for the therapy of infectious diseases. Clinical trials conducted over the last decades confirmed the therapeutic potential of phages, but more data is needed for reliable clinical application. Phage application protocols must move towards a logical operating framework. Ideally, these developments should be classified as standard and universal.
Not applicable.
None.
Conflicts of Interest
The authors declared that there is no conflict of interest.
Received: 2021/09/4 | Accepted: 2022/01/8 | ePublished: 2022/03/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |