year 13, Issue 3 (July - August 2019)
Iran J Med Microbiol 2019, 13(3): 180-193 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Faraji Kafshgari S, Maghsoodlou Y, Khomeiri M, kashiry M, Babaei A. Isolation of Escherichia coli specific Lytic Phages from Waste Water and evaluation of its antimicrobial effect In Vitro and Chicken meat. Iran J Med Microbiol 2019; 13 (3) :180-193
URL: http://ijmm.ir/article-1-951-en.html
URL: http://ijmm.ir/article-1-951-en.html
Samaneh Faraji Kafshgari1 
 , Yahya Maghsoodlou2
, Yahya Maghsoodlou2 
 , Morteza Khomeiri1
, Morteza Khomeiri1 
 , Mahboobeh Kashiry1
, Mahboobeh Kashiry1 
 , Arash Babaei3
, Arash Babaei3 


 , Yahya Maghsoodlou2
, Yahya Maghsoodlou2 
 , Morteza Khomeiri1
, Morteza Khomeiri1 
 , Mahboobeh Kashiry1
, Mahboobeh Kashiry1 
 , Arash Babaei3
, Arash Babaei3 

1- Department of Food Science and Technology, Faculty of Food Industries, University of Agricultural Sciences and Natural Resources, Gorgan, Iran
2- Department of Food Science and Technology, Faculty of Food Industries, University of Agricultural Sciences and Natural Resources, Gorgan, Iran ,y.maghsoodlou@au.ac.ir
3- Department of Biology, Faculty of Basic Sciences, Malayer University, Malayer, Iran
2- Department of Food Science and Technology, Faculty of Food Industries, University of Agricultural Sciences and Natural Resources, Gorgan, Iran ,
3- Department of Biology, Faculty of Basic Sciences, Malayer University, Malayer, Iran
Full-Text [PDF 1625 kb]
(2880 Downloads)
| Abstract (HTML) (6039 Views)
Escherichia coli is one of the most important contaminants in chicken meat causing urinary tract infection, septicemia, neonatal meningitis and traveler’s diarrhea as its most common complications (3,4). Therefore, it is necessary to develop new methods for the detection and control of this bacterium (5). Bacteriophages are bacterial specific viruses that have direct effect on target cells and specific function and do not infect eukaryotic cells as their advantages (10). On the other hand, their disadvantages are phage resistance, the possibility of virulent phage mutation and transformation to lysogenic phage (11). The aim of this study was to isolate the E. coli specific lytic phage and identify its possible family as well as to evaluate its efficacy to reduce E. coli in chicken meat.
E. coli bacteria PTCC (1330) and ATCC (33876, 35218, 25922), DHα5 (SCC: 1785) and BL21 (PTA: 5976); Staphylococcus aurous (ATCC: 2392), Yersinia enterocolitica (PTCC: 1785) and Salmonella enterica (ATCC: 14028) were used in this study.
The number of phage particles=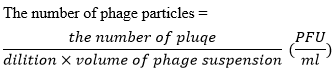
Table 2
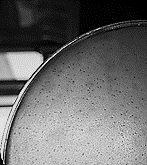
Fig 1. The effect of isolated lytic phage on Escherichia coli and plaque formation
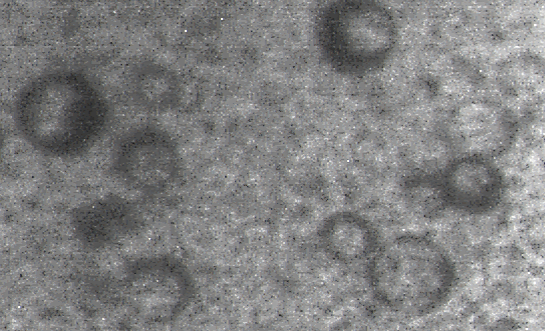
Fig 2. TEM of Escherichia coli lytic phage structure

The aim of this study was to isolate, identify and evaluate the antimicrobial effect of E. coli specific lytic phage in chicken meat. The number of isolated phage particles was estimated to be. 108 to 1010 PFU/mL. The isolated phage had anti-microbial effect against 5 out of the 6 E. coli strains tested and had relatively broad host range and was probably a member of the Tectiviridae family. In this study, the use of E. coli specific lytic phage (108 PFU/mL) in chicken meat decreased 1.2 log in the first 24 h after inoculation, and continued with lower speed to 48 h and then became almost constant, so that on the fourth day after inoculation, E. coli population reached approximately less than 1 log cycle. Generally, most of the discovered phages in the world have long or short tail and belong to the podoviridae, myoviridae and syphoviridae families and little information is available on the isolation and identification of tailless phages. Chai et al. (2016) isolated phage φHN161 from the wastewater that was tailless and had a spherical capsid which belonged to the Tectiviridae family. It had the potential to destroy E. coli O161. Moreover, the isolated phage in the present study was morphologically similar to the phage discovered by these researchers and therefore it is likely that it also belongs to the Tectiviridae family (30). Fiorentin et al. (2005) used lytic phages of S. enteritidis (109 PFU/mL) to reduce the count number of S. enteritidis (106 CFU/mL) inoculated into chicken skin. According to their results, a significant decrease in the number of Salmonella bacteria was observed in the samples treated with phage after 3, 6 and 9 days (32). The results of this study also showed a significant decrease in the population of E. coli inoculated into chicken meat after the phage treatment.
In this study, the isolated E. coli specific lytic phage from wastewater was tailless and had spherical capsid possibly belonging to the Tectiviridae family. This phage had antimicrobial effect on 4 out of the 5 Escherichia coli strains tested in this study but had no antimicrobial effect on S. enterica, Yersinia enterocolitica and S. aureus species. It also reduced the amount of chicken meat inoculated E. coli from 3 log to 1.8 log after 24 h and to less than 1 log cycle after 4 days. Therefore, it can be used as an E. coli biocontrol agent in foods.
The authors of this article thank of the department of Food Science and Engineering, Faculty of Food Industries, University of Agricultural Sciences and Natural Resources, and the department of Biology, University of Malayer.
Full-Text: (6912 Views)
Introduction
Escherichia coli is one of the most important contaminants in chicken meat causing urinary tract infection, septicemia, neonatal meningitis and traveler’s diarrhea as its most common complications (3,4). Therefore, it is necessary to develop new methods for the detection and control of this bacterium (5). Bacteriophages are bacterial specific viruses that have direct effect on target cells and specific function and do not infect eukaryotic cells as their advantages (10). On the other hand, their disadvantages are phage resistance, the possibility of virulent phage mutation and transformation to lysogenic phage (11). The aim of this study was to isolate the E. coli specific lytic phage and identify its possible family as well as to evaluate its efficacy to reduce E. coli in chicken meat.
Materials and Methods
E. coli bacteria PTCC (1330) and ATCC (33876, 35218, 25922), DHα5 (SCC: 1785) and BL21 (PTA: 5976); Staphylococcus aurous (ATCC: 2392), Yersinia enterocolitica (PTCC: 1785) and Salmonella enterica (ATCC: 14028) were used in this study.
Isolation, Purification and Storage of Phage
A total of 100 mL of wastewater was mixed with 50 mL of LB liquid medium, into which the host bacterium was inoculated. After incubation at 37°C and shaking in 100 rpm for 24 h, 50 mL of this solution was centrifuged for 15 min and the supernatant was then filtered with a 0.45 nm filter and then serial dilution was prepared. Then 100 µL of dilutions 6, 7 and 8 were mixed with 2.5 mL of melted LB agar medium and 200 µL primary bacteria and incubated in LB agar plate for 24 hours at 37°C. The lytic plaque with the same shape and size were selected and mixed with 500 µL LB medium and incubated for 15 minutes after which the serial dilution was prepared. 100 µL of each microtube was removed and mixed with 200 µL of the primary bacteria and 2.5 mL of LB agar medium and poured into LB agar plate and incubated at 37°C for 24 hours. From the stock phage in the broth, 500 μL was removed and mixed with 500 μL of sterile glycerol in the microtube. Phage storage was performed at -70°C (15).Morphology and Detection of Phage Family by Transmission Electron Microscopy (TEM)
TEM imaging was performed to investigate of morphology of the isolated phage. The TEM images were matched with previously identified standard phages to identify their families (16).Evaluation of Antimicrobial Efficacy of the Isolated Phage in vitro
After activation of Escherichia coli in LB medium, 100 µL of this suspension was added to 5 mL of semi-solid LB medium and then transferred to a plate and gave 15 minutes time to the medium to harden. The serial dilution was then prepared from phage and 10 μL of the phage solution was added to the surface of activated bacteria and spread on the plate surface and incubated for 24 hours at 37°C. The number of phage particles in the suspension was determined using the following equation (17).The number of phage particles=
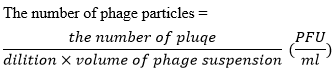
Host Spectrum and Phage Specificity
Host spectrum and specificity of phage against 5 different strains of E. coli as well as S. enterica, Yersinia enterocolitica and Staphylococcus aureus species were evaluated by plaque assay (18).Evaluation of the Isolated Phage Efficiency in Reducing Escherichia coli in Chicken Meat
A total of 25 g of chicken fillet was sterilized by gamma irradiation (KG 10) (19) and immersed into 100 mL of bacterial suspension (103 CFU/g). Phage solution (108 PFU/mL) was also added. Counting of living bacteria was immediately done in all samples after the addition of bacteria and phage (20).Results
Isolation and Determination of Phage Particles
Between 108 and 1010 PFU/mL of E. coli specific phage was isolated from the wastewater which appeared as clear zones (lysed areas) in the culture medium.Phage Morphology and Identification of Its Possible Family
The isolated phage was tailless and had a spherical capsid or head about 70-60 nm, and was probably a member of the Tectiviridae family (Fig 2).Phage Specificity and Host Spectrum
Among of the 5 selected strains, the isolated phage had antimicrobial effect on the 4 out of the 5 tested strains of E. coli whilst had no antimicrobial effect on the other species tested in this article (Table 1).| Plaque | Bacteria |
| + | E. coli PTCC 1330 |
| - | Salmonella enterica subsp. Enterica serovar typhimurium ATCC 14028 |
| - | Staphylococcus aureus ATCC 2392 |
| - | Yersinia enterocolitica subsp. enterocolitica PTCC 1785 |
| Plaque | Strains of Escherichia coli | |
| + | 1330 | E. coli PTCC |
| + | 25922 | E. coli ATCC |
| - | 35218 | E. coli ATCC |
| + | 33876 | E. coli ATCC |
| + | 2197 | E.coli DHα5 SCC |
| + | 5976 | E. coli BL21 PTA |
Evaluation of the Isolated Phage Anti-microbial Efficacy in Chicken Meat
The isolated phage reduced the bacterial population in chicken meat by 1.2 log in the first 24 h. The reduction of host bacterial continued with lower speed in other days. So that on the fourth day after inoculation, the E. coli levels reached approximately less than 1 log cycle.Table 2
Fig 1. The effect of isolated lytic phage on Escherichia coli and plaque formation

Fig 2. TEM of Escherichia coli lytic phage structure

Fig 3. Effect of Escherichia coli specific lytic phage on Escherichia coli contamination in chicken
Discussion
The aim of this study was to isolate, identify and evaluate the antimicrobial effect of E. coli specific lytic phage in chicken meat. The number of isolated phage particles was estimated to be. 108 to 1010 PFU/mL. The isolated phage had anti-microbial effect against 5 out of the 6 E. coli strains tested and had relatively broad host range and was probably a member of the Tectiviridae family. In this study, the use of E. coli specific lytic phage (108 PFU/mL) in chicken meat decreased 1.2 log in the first 24 h after inoculation, and continued with lower speed to 48 h and then became almost constant, so that on the fourth day after inoculation, E. coli population reached approximately less than 1 log cycle. Generally, most of the discovered phages in the world have long or short tail and belong to the podoviridae, myoviridae and syphoviridae families and little information is available on the isolation and identification of tailless phages. Chai et al. (2016) isolated phage φHN161 from the wastewater that was tailless and had a spherical capsid which belonged to the Tectiviridae family. It had the potential to destroy E. coli O161. Moreover, the isolated phage in the present study was morphologically similar to the phage discovered by these researchers and therefore it is likely that it also belongs to the Tectiviridae family (30). Fiorentin et al. (2005) used lytic phages of S. enteritidis (109 PFU/mL) to reduce the count number of S. enteritidis (106 CFU/mL) inoculated into chicken skin. According to their results, a significant decrease in the number of Salmonella bacteria was observed in the samples treated with phage after 3, 6 and 9 days (32). The results of this study also showed a significant decrease in the population of E. coli inoculated into chicken meat after the phage treatment.
Conclusion
In this study, the isolated E. coli specific lytic phage from wastewater was tailless and had spherical capsid possibly belonging to the Tectiviridae family. This phage had antimicrobial effect on 4 out of the 5 Escherichia coli strains tested in this study but had no antimicrobial effect on S. enterica, Yersinia enterocolitica and S. aureus species. It also reduced the amount of chicken meat inoculated E. coli from 3 log to 1.8 log after 24 h and to less than 1 log cycle after 4 days. Therefore, it can be used as an E. coli biocontrol agent in foods.
Acknowledgments
The authors of this article thank of the department of Food Science and Engineering, Faculty of Food Industries, University of Agricultural Sciences and Natural Resources, and the department of Biology, University of Malayer.
The authors reported no conflict of interest.
Type of Study: Original Research Article |
Subject:
Antimicrobial Substances
Received: 2019/08/1 | Accepted: 2019/11/22 | ePublished: 2019/11/22
Received: 2019/08/1 | Accepted: 2019/11/22 | ePublished: 2019/11/22
References
1. Atterbury RJ, Dillon E, Swift C, Connerton PL, Frost JA, Dodd CER, et al. Correlation of Campylobacter bacteriophage with reduced presence of hosts in broiler chicken ceca. Appl Environ Microbiol. 2005; 71(8):4885-7. [DOI:10.1128/AEM.71.8.4885-4887.2005] [PMID] [PMCID]
2. Muth, M K, Fahimi M, Karns SA. Analysis of Salmonella control performance in U.S. young chicken slaughter and pork slaughter establishments. J of Food Protec. 2009; 72(1):6-13. [DOI:10.4315/0362-028X-72.1.6] [PMID]
3. Motarjemi Y, Moy GG, Jooste PJ, Anelich LE. Food Safety Management. In: Motarjemi Y, Lelieveld H (Eds). San Diego: Academic Press; 2014. [DOI:10.1016/B978-0-12-381504-0.00041-X]
4. Hosseini Jazani N, Hadizadeh O, Farzaneh H, Moloudizargari M. Synergistic antibacterial effects of β- Chloro- L- alanine and phosphomycin on urinary tract isolates of E. coli. Bio J Microbiol. 2013; 1(4):1- 6.
5. Hill B, Smythe B, Lindsay D, Shepherd J. Microbiology of raw milk in New Zealand. Int J Food Microbiol. 2012; 157(2):305-308. [DOI:10.1016/j.ijfoodmicro.2012.03.031] [PMID]
6. Kutter E, Sulakvelidze A (Eds). Bacteriophages: Boca Raton: CRC Press; 2005; 1-5. [DOI:10.1201/9780203491751]
7. World Health Organization. The FTY eighth world health assembly. Geneva: WHO; 2005.
8. World Health Organization. Food safety & food-borne illness. fact sheet no. 237 (reviewed March 2007). Geneva: WHO; 2007.
9. Steinbacher S, Baxa U, Miller S, Weintraub A, Seckler R, Huber R. Crystal structure of phage P22 tails pike protein complexed with Salmonella sp. antigen receptors. Proc Natl Acad Sci USA. 1996; (93):10584-8. [DOI:10.1073/pnas.93.20.10584] [PMID] [PMCID]
10. Kutateladze M, Adamia R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 2010; (28):591-5. [DOI:10.1016/j.tibtech.2010.08.001] [PMID]
11. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis. 2011; 17(1):7-15.
https://doi.org/10.3201/eid1701.P21101 [DOI:10.3201/eid1701.P11101] [PMID]
12. Pourmahmoodi A, Mohammadi J, Mirzai A, Momeni Negad M, Afshar R. Epidemiological study of traditional ice cream in Yasuj. Armaghan Danesh. 2002; 8(29):59-65. [Persian]
13. Whichard JM, Sriranganathan N, Pierson FW. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquidculture and on chicken frankfurters. J of Food Prot. 2003; (66):220-5. [DOI:10.4315/0362-028X-66.2.220] [PMID]
14. Ranjbar M, Sharifiyan A, Shabani Sh, Amin Afshar M. Antimicrobial effect of garlic extract Staphylococcus aureus and Escherichia coli bacteria in a cook ready chicken to meal model. Food Technol Nutr. 2014; 11(4):57-68.
15. Zare1 L, Shenagari M, Mirzaei MKH, Mojtahedi A. Isolation of lytic phages against pathogenic E.coli isolated from diabtic ulcers. Iran J Med Microbiol. 2018; 11(2):34-41.
16. Borysowski J, Weberdabrowska B, Gorski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med. 2006; (231):366-77. [DOI:10.1177/153537020623100402] [PMID]
17. Vonasek E, Phuong L, Nitin N. Encapsulation of bacteriophages in whey protein films for extended storage and release. Food Hydro. 2014; (37):7-13. [DOI:10.1016/j.foodhyd.2013.09.017]
18. Soltan Dallal MM, Imeni SM, Nikkhahi F, Rajabi Z, Salas SP. Isolation of E. Coli bacteriophage from raw sewage and comparing its antibacterial effect with ceftriaxone antibiotic. Int J Adv Biotechnol Res. 2016; 7(3):385-91.
19. Hungaro HM, Mendonca RCS, Gouvea DM, Vanetti MCD, Pinto CLD. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res Int. 2013; (52):75-81. [DOI:10.1016/j.foodres.2013.02.032]
20. Anany H, Chen W, Pelton R, Griffiths MW. Biocontrol of Listeria monocytogenes and Escherichia Coli O157: H7 in meat by using phages immobilized on modified cellulose membranes. Appl Environ Microbiol. 2011; (77):6379-87. [DOI:10.1128/AEM.05493-11] [PMID] [PMCID]
21. Hagens S, Loessner MJ. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Current Pharma Biotech. 2010; (11): 58-68. [DOI:10.2174/138920110790725429] [PMID]
22. Hooton S, Atterbury RJ, Connerton IF. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. International Journal of Food Microbiology . 2011; (151): 157-163. [DOI:10.1016/j.ijfoodmicro.2011.08.015] [PMID]
23. Bigwood T, Hudson JA, Billington C. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS microbiol letters.2009; 291: 59-64. [DOI:10.1111/j.1574-6968.2008.01435.x] [PMID]
24. Greer GG. Bacteriophage control of foodborne bacteria. J of Food Prot. 2005; (68): 1334-1334 [DOI:10.4315/0362-028X-68.5.1102] [PMID]
25. Merabishvili M, Pirnay J, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V, Mast J, Van Parys L. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. 2009; PloS one 4, e4944. [DOI:10.1371/journal.pone.0004944] [PMID] [PMCID]
26. Carvalho CM, Santos SB, Kropinski AM, Ferreira EC, Azeredo J. Phages as therapeutic tools to control major foodborne pathogens: Campylobacter and Salmonella, In Bacteriophages. 2012. Croatia: InTech, pp 179-214.
27. Singh V, Jain P, Dahiya S. Isolation and characterization of bacteriophage from waste water against E.coli, a food born pathogen. Microbiol Biotech. 2016; (1):163-70.
28. Jann K, Schmidt G, Wallenfels B. Isolation and Characterization of Escherichia coli bacteriophage Ω 8 specific for E. coli strains belonging to sero-group Ω 8. General Microbiol. 1971; (67):289-97. [DOI:10.1099/00221287-67-3-289] [PMID]
29. Beheshti Maal K, Soleimani Delfan A, Salmanizadeh SH. Isolation and identification of two novel Escherichia Coli bacteriophages and their application in wastewater treatment and coliform's phage therapy. Jundishapur J Microbiol. 2015; 8(3):e14945. [DOI:10.5812/jjm.14945] [PMID] [PMCID]
30. Chai Q, Dandan W, Liu F, Song F, Tang X, Cao Y, et al. Therapy potential of tailless bacteriophage ΦHN161 and its ability in modulating inflammation caused by bacterial disease. Vet Med Open. 2016; 1(2):36-42. 31. [DOI:10.17140/VMOJ-1-107]
31. Hagens S, Loessner MJ. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr Pharm Biotechnol. 2010; (11):58-68. [DOI:10.2174/138920110790725429] [PMID]
32. FiorentinL, Vieira ND, Barioni Junior W. Use of lytic bacteriophages to reduce Salmonella Enteritidis in Experimentally Contaminated Chicken Cuts. Br J Poultry Sci. 2005; 7(4):255-60. [DOI:10.1590/S1516-635X2005000400010]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





