BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-978-en.html
2- Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran ,
.
Today, drug resistance in many bacteria is significantly increased. Bacterial resistance to antibiotics is one of the biggest challenges that threatens human health in the modern age (1). Prevention of food spoilage and control of food poisoning pathogens are usually done with preservatives that have harmful effects on human health or increase the resistance of microbes to antimicrobial agents such as antibiotics (2,3). Nanoparticle technology is a new technology that is sweeping the whole world, and more precisely, nanotechnology is not part of the future, but it is the future. Nanotechnology is not a new discipline, but rather a new approach in all disciplines for which they have listed applications in the various fields of food, medicine, medical diagnosis and industry (4). The nanoparticles are about 1 to 100 nm in diameter and have unique surface composition, size, shape, and chemical properties. The fine particle design can be used to target germs (5). These particles have been widely used as antimicrobial agents for the control and treatment of microbes and this may suggest the use of nanoparticles for antibiotic replacement (6). Nanoparticles have antibacterial properties due to their high surface-to-volume ratio and small size, penetrating microorganisms, as well as having photocatalytic, catalytic and ionic effects, and are widely used in the fight against pathogenic microorganisms such as bacteria, fungi, and viruses (7,8). MgO nanoparticles of non-inorganic metal oxides have antibacterial properties and their important advantages are their non-toxicity and rapid, easy and low cost synthesis. The nanoparticle has been identified as a safe substance by the US Food and Drug Administration (21CFR184.1431) (9, 10). Bacteria such as Staphylococcus aureus, Bacillus cereus and Salmonella enterica play an important role in food poisoning (11). Enterotoxins of B. cereus cause diarrhea and nausea. It grows in foods such as rice, cereals, dairy and meat and by producing two types of enterotoxins, it causes food poisoning and remains in pasteurized milkdue to the presence of spores as an opportunistic agent (12,13). In addition, Salmonella species, like S. enterica, are currently the most common cause of food poisoning (14). The aim of this study was to synthesize MgO nanoparticles and investigate their antibacterial effects against three food poisoning causing bacteria.
Synthesis and Characterization of MgO Nanoparticles
Chemical deposition method was used to prepare magnesium oxide nanoparticles (15,16). Therefore, 0.21 g magnesium nitrate and 2 mmol (0.44 g) sodium hydroxide was heated in a 100 mL human under stirring conditions at 60°C for 45 min. In another flask, 4 mmol (0.30 g) of sodium dodecyl sulfate surfactant as a stabilizing source in 10 mL methanol solvent was placed on a magnetic stirrer at 60°C for 30 min at a pH of 8 to 9. The magnesium oxide solution was then mixed with a surfactant solution in a 100 mL Erlenmeyer flask and incubated at 60°C for one hour. The resulting solution was first placed in an ultrasonic bath at 60 watts and then placed at 60°C to stabilize the particles. After deposition, the solution was discarded to remove the precipitate from the reaction solution. To remove impurities, the precipitate was washed several times with double distilled water and ethanol, and dried in a vacuum oven at 60°C for 24 hours. Quality control and morphology of magnesium oxide nanoparticles made by XRD (X-ray diffraction) and SEM (Scanning electron microscope) methods were used (15,16).
Antibacterial Effects of MgO Nanoparticles
Antibacterial effects of magnesium oxide nanoparticles against three food poisoning bacteria including Staphylococcus aureus, Bacillus cereus and Salmonella enterica isolated from food samples were done by Agar well diffusion method (17). Different concentrations of 5, 10, 20, 40, 2.5, 1.25, 0.62, 0.31 and 0.15 mg/mL of magnesium nano oxide made in dimethyl sulfoxide and methanol (v/v) solvents was prepared by doubling the dilution in each step (18). Each bacterium was prepared from a standard suspension equivalent to a 0.5 McFarland's solution in sterile normal saline. The wells were embedded in a 4 mm diameter well in Muller Hinton agar medium and 20 μL of each nanoparticle concentration was poured into each well. This was done at concentrations of 5,10,20,40, 2,5, 1/25, 0.62, 0.31 and 0.15 mg / ml of magnesium nano oxide were performed separately with three replications. Plates were incubated at 4°C for one hour, and after absorbing the nano solution into the culture medium, they were incubated in the oven at 37°C for 24 h. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the synthesized nanoparticles against all three bacteria were determined. After incubation for 24 hours at 37°C, the diameter inhibition zone was measured in millimeters. It should be noted that solvent dimethyl sulfoxide and methanol were used as negative control. All the media and chemicals used were provided by Merck German Company (19).
Determination of Antibiotic Resistance Pattern of Bacteria
The purpose of the antibiogram was to determine the antibiotic resistance pattern of the bacteria used from wild strains isolated from food. For this purpose, disk diffusion method was used (20). A standard concentration of 0.5 standard McFarland was prepared from each bacterium Antibiotics of cefalexin (30 mcg), amikacin (30 mcg), neurofloxacin (10 mcg), ciprofloxacin (5 mcg), gentamicin (10 mcg), ampicillin (20 mcg), sulfamethoxazole (10 mcg) and amoxicillin (25 mcg), made in Pars Tab Company, Iran. After incubation for 24 h at 37°C, the diameter of inhibition zone was measured in mm.
Results of Quality of Synthesized MgO Nanoparticles
Identification and analysis of samples of MgO Nanoparticles made by XRD and SEM methods showed that these particles were spherical in shape with a diameter of about 64.23 nm and were well dispersed (Figure 1).
X-ray peaks also showed that the sample was highly purified in the crystalline phase. The X-ray pattern obtained from magnesium oxide microstructures shows that the structure in question is high purity without being amorphous with the JCPDS structural code. 01-1235 is formed (Figure 2).
The MgO nanoparticles synthesized against all three S. aureus, B. cereus and S. enterica were effective at low concentrations based on the results shown in Figure 3.
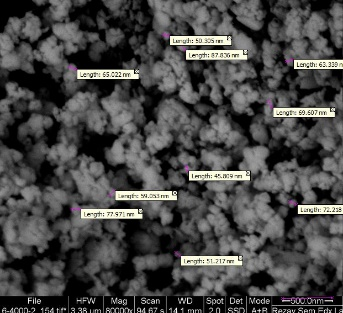
Figure 1. Observation of magnesium oxide nanoparticles made by scanning electron microscopy (SEM)
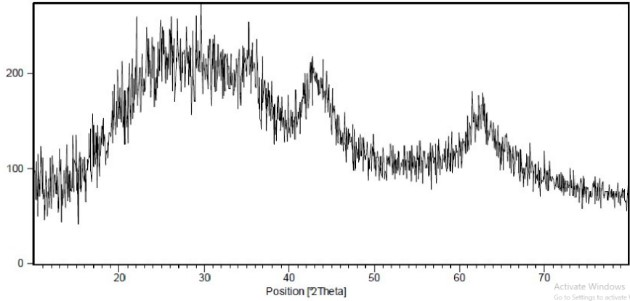
Figure 2. X-ray diffraction pattern of MgO nanoparticles Antibacterial Properties of MgO Nanoparticles

Figure 3. MIC and MBC of MgO nanoparticles against 3 bacteria
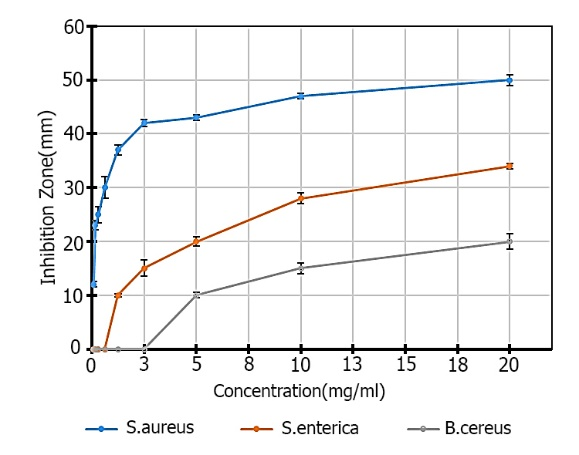
Figure 4. Comparison of the average diameter of inhibition zone (mm) based on the concentration of nanoparticles (mg / mL)
The inhibition zone diameter increases with increasing concentration of magnesium oxide nanoparticles. Table 1 shows the mean diameter of non-growth zone of magnesium oxide nanoparticles compared to the three bacteria. The results of analysis of variance of mean inhibition zone (mm) diameter based on concentration of nanoparticles (mg/mL) are shown in Table 2.
Table 1. Average inhibition zone diameter (mm) based on concentration of nanoparticles (mg/mL)

Table 2. Variance analysis of diameter average of inhibition zone (mm) based on nanoparticles concentration (mg/ mL)

Minimum Inhibitory Concentration (MIC) of S. aureus, S. enterica and B. cereus were 0.075, 1.25 and 5 mg/mL, respectively, and Minimum Bactericidal Concentration (MBC) of Magnesium Oxide Nanoparticles were 0.15, 2.5 and 10 mg/mL respectively (Figures 3 and 4). Comparison of mean inhibition zone diameter based on Duncan's method at α=0.05 showed that the concentrations used on average zone diameter were significantly different for S. aureus up to concentration 10 and for bacteria. Others are significant up to a concentration of 20 mg/ mL (Table 3).
Table 3. Comparison of mean diameter of inhibition zone (mm) based on concentration of nanoparticles (mg/ mL) by Duncan method at α = 0.05.
| B. cereus | S. enterica | S. aureus | Concentration |
| a | a | a | 20 |
| b | b | a | 10 |
| c | c | b | 5 |
| d | d | b | 2.5 |
| d | e | c | 1.25 |
| d | f | d | 0.62 |
| d | f | e | 0.31 |
| d | f | e | 0.15 |
| d | f | f | 0.075 |
| Similar letters in each group did not differ significantly. | |||
Antibiogram Results
The results of the antibiotic effect on three bacterial strains isolated from the studied samples showed that Staphylococcus aureus was sensitive to only two antibiotics cefalexin and amikacin and to neurofloxacin, sephroploxacin, gentamicin, Sulfamethoxazole and amoxicillin were resistant. Resistance to cephalexin, neurofloxacin and sulfamethoxazole was observed in S. enterica and resistance to cephalexin, sulfamethoxazole, amoxicillin was observed in Bacillus cereus.
The antimicrobial effect of the nanoparticles is due to their effect on the cell wall and the outer part of the bacterial cell and also because of its entry into the cell and its effect on the different internal parts of the bacterial cell. The nanoparticles initially absorb the negative charge on the outer surface of the bacterium due to the positive electron charge. This binding can both disrupt the bacterial electrolyte balance and can affect the respiratory cycle of the bacterial cells. In the latter case, the nanoparticles affect the proteins and DNA, the respiratory chain enzymes responsible for cell growth and disruption of replication, resulting in the formation of free radicals and reactive oxygen (21,22). Synthesis of nanoparticles with the potential to inhibit the growth of germs called nano-antibiotics is of great importance (23,24). The size of the nanoparticles is very effective in their antimicrobial activity and the smaller the size, the greater the antimicrobial effect is, plus the bacterial strain is effective in the sensitivity of the nanoparticles (25). In the present study, the antibacterial effects of magnesium oxide nanoparticles were chemically evaluated against three food poisoning bacteria and all of them were resistant to some antibiotics but even on magnesium oxide nanoparticles. The concentrations were very low. Similar research has been done by other researchers on the effects of magnesium nanoparticles. The mechanism of antibacterial activity of magnesium oxide nanoparticles against Escherichia coli and Pseudomonas aeruginosa and S. aureus. The minimum inhibitory concentrations were 0.5 mg / mL for E. coli and 1 mg / mL for P. aeruginosa and S. aureus. They recognized that the sensitivity of the bacteria to the nanoparticles was not only related to the cell wall structure but also to the lipid peroxidation and production of reactive oxygen species (26). Other researchers examined the antibacterial effect of magnesium oxide nanoparticles, with an average size of 20 nm, on a number of bacteria, including E. coli, Salmonella and Campylobacter. Minimum inhibitory concentrations for E. coli, Salmonella and Campylobacter were 1, 2 and 0.5 mg/mL, respectively. At 2 mg/mL concentration Campylobacter was completely inhibited for 2 h and at 2 to 4 mg/mL growth of E. coli and Salmonella stopped for 6-8 h (27). In another study, the magnesium oxide nanoparticles and the natural antimicrobial substance polyelelisine had antimicrobial effects on two foodborne bacteria, E. coli 0157: H7 (35218 ATCC) and Listeria monocytogenes (ATCC 19118), and their use had a synergistic effect. It also reduced the minimum concentration of the two compounds (28). A study by Ansari Moghaddam et al., aimed to determine the cytotoxicity of magnesium oxide nanoparticles on 562 K leukemia K cells, showing that different concentrations of Mgo nanoparticles had no effect on normal and even carcinogen cells. An increase in the concentration of nanoparticles is not correlated with an increase in its effect on the cell lines studied. Therefore, magnesium oxide nanoparticles have no cytotoxic effect on human cells and have specific effects on microbial cells (29).The findings of the present study and other researchers indicated that magnesium oxide metal nanoparticles have very effective antibacterial properties and there is a direct relationship between the concentration of nanoparticles and the bacterial elimination rate and the antibacterial effect is a bactericidal effect. Magnesium oxide nanoparticles have no growth inhibitory (bacteriostatic) effects, and the nanoparticle solution appears to be diffused as microscopic particles and can easily penetrate into bacterial cells. In general, nanoparticles can also be one of the important pollutants in the toxicity of different parts of the body due to their physical and chemical properties as well as the positive properties seen in in vitro conditions. Assessing the type of exposure and identifying hazardous properties for nanoparticles requires consideration of safety and knowledge of the effects of these substances on humans and the environment, thorough understanding of the substances and their toxicological effects. Nanoparticles, like a double-edged sword, have beneficial and harmful effects. Although nanotechnology has revolutionized and expanded widely in many fields, however, reducing toxicity and the dangers of exposure to nanoparticles should be one of the main goals of this field.
Due to the antimicrobial effect that magnesium oxide nanoparticles have on food-borne bacteria, it can be considered as a good candidate for controlling microbial contamination of food in food antimicrobial packaging.
Hereby we express our gratitude to our colleagues at the Microbiology Research Laboratory, Islamic Azad University, Kerman Branch.
Authors declared no conflict of interests.
Received: 2019/10/19 | Accepted: 2020/03/4 | ePublished: 2020/03/4
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







