BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2462-en.html


 , Shomaia Yasmin Mitu1
, Shomaia Yasmin Mitu1 

 , Md. Shamsul Arefin1
, Md. Shamsul Arefin1 

 , Meftahul Jannat Mitu1
, Meftahul Jannat Mitu1 

 , Md. Sabir Hossain2
, Md. Sabir Hossain2 

 , Salequl Islam1
, Salequl Islam1 

 , M.A. Karim Rumi3
, M.A. Karim Rumi3 

 , M. Hasibur Rahman4
, M. Hasibur Rahman4 


2- Department of Biochemistry and Molecular Biology, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh
3- Department of Pathology and Laboratory Medicine, University of Kansas Medical Center, Kansas City, KS 66160, USA
4- Department of Microbiology, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh ,
Pseudomonas aeruginosa (P. aeruginosa) is one of the most common pathogens associated with nosocomial infections (1). This Gram-negative bacterium is a member of the infamous ESKAPE group of bacteria, known for their rapidly increasing antibiotic resistance in healthcare facilities (2, 3). The global priority list of emerging antibiotic-resistant bacteria recognizes P. aeruginosa as a critical pathogen that requires an in-depth study (4). It is commonly associated with wound infections, urinary tract infections (UTIs) (particularly catheter-associated UTIs), ventilator-associated respiratory tract infections (RTI), and cystic fibrosis (1, 5).
The carbapenem group of antibiotics (imipenem, meropenem, ertrapenem, and doripenem) is clinically effective against P. aeruginosa (5, 6). However, a steep rise in carbapenem resistance has been reported over the last few years (7). Susceptibility to carbapenems is dependent on their entry to bacteria through the outer membrane porin protein OprD (8). Thus, a reduced expression of OprD can lead to carbapenem resistance in P. aeruginosa (8). Other mechanisms of carbapenem resistance include decreased permeability of outer membrane porins, increased production of molecular efflux pumps, acquisition of chromosomal cephalosporinases, and expression of broad-spectrum beta-lactamases, especially carbapenemases like metallo-β-lactamases (MBLs) (9). Acquisition of MBLs through horizontal gene transfer has been increasingly identified in Gram-negative isolates from hospitalized patients (10). These enzymes can hydrolyze most of the β-lactams and are not repressed by serine β-lactamase repressors like tazobactam and clavulanates (11). As MBLs depend on bivalent zinc ions for the hydrolysis of β-lactam antibiotics, they are inactivated by metal chelators like EDTA (12).
The origins of MBLs remain unclear; it is most likely transferred from environmental bacteria like Enterobacteriaceae (13). The genes encoding MBLs are mainly spread through mobile genetic elements like integrons residing on plasmids or bacterial genomic DNA (13, 14). The majority of MBLs belong to subclass B1 (15, 16) and the 3 most common MBLs, namely IMP (imipenemase), VIM (Verona integron-encoded Metallo-β-lactamase), and NDM (New Delhi Metallo-β-lactamase) are well-known for their epidemiological and clinical importance (12, 13, 17). The bla-VIM was first detected in P. aeruginosa in European countries during the late 1990s, and more than 20 different VIM allotypes have been identified worldwide (13, 18). However, bla-NDM-1, which was detected in K. pneumoniae in 2008, has been widely disseminated among Enterobacteriaceae in India (14). A significant increase in prevalence of these MBLs has been seen throughout the world, including the United Kingdom and Southeast-Asian countries like India (14), Pakistan (19), and Bangladesh(20).
In this study, we investigated the presence of carbapenem-resistant P. aeruginosa infection among hospitalized patients in Dhaka, Bangladesh. We also aimed to analyze the presence of different MBL-encoding genes like bla-IMP, bla-VIM, bla-SPM and bla-NDM-1 to deduce the role of MBL variants in carbapenem-resistance of P. aeruginosa. The possible epidemiological association or link among the isolates was also explored by Enterobacterial Repetitive Intergenic Consensus-Polymerase Chain Reaction (ERIC-PCR) approach. We observed that resistance to carbapenems has been doubled in the past decade (6). Such a rapid increase in carbapenem resistance was mediated by MBL. We also detected the expression of MBL-variants in carbapenem resistant P. aeruginosa isolates and determined the heterogenic nature of the clinical isolates.
Clinical samples were collected from patients admitted to two academic hospitals, Enam Medical College Hospital (EMCH) and Gonoshasthaya Samaj Vittik Medical College Hospital (GMCH) located at Savar, Dhaka, Bangladesh from April 2020 to January 2021. The presence of P. aeruginosa was studied in 238 samples consisting of 115 midstream urine, 69 pus, 21 secondary wound infection swab, 12 urinary catheter swab, 10 burn wounds, 9 blood, and 2 tracheal aspirate specimens. Pseudomonas Cetrimide Agar (Scharlab S. L., Spain) was used to determine the colony morphology, fluorescence, and pigment production, followed by conventional biochemical tests according to Bargey’s Manual of Systemic Bacteriology for presumptive identification of P. aeruginosa as described previously (21, 22). P. aeruginosa ATCC 27853 was used as a positive control. Selected isolates were stored in glycerol broth at -80°C.
2.2 Molecular Identification
Molecular identification was carried out by sequencing 16S rDNA of 14 representative isolates. DNA templates prepared from the selected isolates were subjected to polymerase chain reaction (PCR) (23). PCR products were purified using Promega Wizard SV Gel and PCR Clean-up System (USA) and sequenced. The identity of the isolates was confirmed by BLAST analysis.
2.3. Screening of Carbapenem Resistance
To determine the susceptibility of clinical P. aeruginosa isolates to carbapenem antibiotics, Kirby Bauer disk diffusion test was used (24), followed by determination of minimum inhibitory concentration (MIC) against imipenem (IMP 10 µg) and meropenem (MPM 10 µg). Antibiotic disks were obtained from Oxoid, UK. P. aeruginosa PAO1 was used as control. The results were interpreted according to CLSI standards and guidelines (25). Multidrug resistant (MDR) nature of isolates was observed against 9 additional antibiotics of 6 different groups (data not included in this study) and interpreted accordingly (26, 27).
2.4. Phenotypic Detection of MBL Production
To detect the production of MBL, imipenem-resistant isolates were subjected to IMP-EDTA double disk synergistic test. The pure cultures of selected isolates were plated on Mueller-Hinton agar (Oxoid, UK) to prepare bacterial lawn. Two imipenem (IMP 10 µg) disks were placed on prepared lawn by at least 20 mm distance. One of the disks was impregnated with 0.5M EDTA to achieve 750 µg/disk concentrations, which can act on MBL by removing zinc ions from the active site of the enzyme, effectively nullifying its activity. The plates were incubated overnight at 37°C, and zone diameters for both IMP and IMP-EDTA disks were measured. Isolates exhibiting ≥17 mm inhibition zones with IMP-EDTA disk were considered MBL-positive, while isolates with ≤14 mm inhibition zones were considered MBL-negative (28).
2.5. PCR Detection of MBL
The presence of genes encoding 4 different variants of MBLs; bla-NDM-1, bla-SPM, bla-IMP, and bla-VIM was investigated in all isolates exhibiting phenotypic resistance against imipenem. PCR reagents were obtained from Promega, USA and the primers were designed according to the previous publications (29, 30). The primers sequences and annealing temperatures are listed in Table 1. For each PCR reaction, 2 µl of prepared bacterial DNA was added to 16 µl of prepared PCR ready-to-use mix containing (1X PCR buffer, 2.5mM MgCl2, 0.25 mM dNTP, 1IU Taq DNA polymerase, 10 pmol of each primer), and deionized water was added to obtain a final volume of 25 µl. The PCR products were visualized using UV transilluminator after electrophoresis on 1% agarose gel.
Table 1. PCR primers specifications.
| Primer | Sequence (5'-3') | Amplicon size (bp) | Annealing Temperature (°C) | Reference |
| bla-IMP | F- GGA ATA GAG TGG CTT AAT TCT C R- CCA AAC CAC TAC GTT ATC T |
188 | 52 | (29) |
| bla-VIM | F- GAT GGT GTT TGG TCG CAT A R- CGA ATG CGC AGC ACC AG |
390 | 52 | (29) |
| bla-SPM | F- AAA ATC TGG GTA CGC AAA CG R- ACA TTA TCC GCT GGA ACA GG |
271 | 52 | (29) |
| bla-NDM-1 | F- CCT ACA ATC TAA CGG CGA CC R- TCG CCG TGT CCA GGT ATA AC |
621 | 56 | (30) |
| ERIC2 | AAG TAA GTG ACT GGG GTG AGC G | ----- | 48 | (31) |
To observe diversity among P. aeruginosa isolates, ERIC-PCR was performed using the primers mentioned elsewhere (31). PCR products were separated on 2% agarose gel and visualized under UV transilluminator. The cluster analysis was conducted using DendroUPGMA (32, 33).
2.7. Plasmid Extraction
Plasmids from carbapenem-resistant P. aeruginosa isolates were extracted according to the hot alkaline lysis method by Kado and Liu (34) with modification. The extracted plasmids were electrophoresed on 0.7% agarose gel with ethidium bromide (0.5 mg/mL) and visualized under UV transilluminator. Reference strains of E. coli K-12 V517 and PDK9 were used as control strains for the extraction method and plasmid size marker (35, 36).
2.8. Statistical Analysis
A validated questionnaire was used for data collection. Collected data were verified and analyzed by IBM SPSS Statistics Data Editor (Version 21). STATA 15 was used for subsequent analysis. P-value of <0.05 was considered significant.
A total of 238 clinical samples were collected from the patients admitted into two academic hospitals in Savar City, at the outskirts of Dhaka, the capital of Bangladesh. Most of clinical samples were collected from patients with UTI, followed by secondary wound infections and abscesses (Table 2). Among the samples, 53 samples (~22%) showed P. aeruginosa growth on selective cetrimide agar, which were further characterized through standard biochemical tests. Identification of the clinical isolates was confirmed by PCR-based 16S rDNA sequence analysis, which showed 95 to 99% identity to P. aeruginosa PAO1 sequence (e-value ≤0). Wound infections were more frequently positive for P. aeruginosa compared to other infections.
3.2. Carbapenem Resistance in Clinical P. aeruginosa Isolates
All 53 positive P. aeruginosa isolates were tested for susceptibility to carbapenem (imipenem and meropenem) using disk diffusion method followed by MIC assay. Out of 53 isolates, 16 (30%) were resistant to both imipenem and meropenem. However, the antibiotic tolerance levels varied widely in MIC assay. While 50% (8/16) of carbapenem-resistant isolates showed moderate resistance (8 µg/ml), the other 50% isolates had much higher resistance level (≥16 to 128 µg/ml). Among 16 carbapenem-resistant P. aeruginosa isolates, 6 were from Gonoshasthaya Samaj Vittik Medical College & Hospital (GMCH) (38%), while the rest 10 (62%) were from Enam Medical College & Hospital (EMCH) (Table 2). Antibiogram profile identified 12 isolates as MDR, and another 2 isolates as extensively drug resistant (XDR). Moreover, uniform resistance against third generation cephalosporins like ceftriaxone and cefotaxime and significantly lower susceptibility to important antibiotics like azithromycin and tetracycline was observed, which is alarmingly higher than previous regional reports (37).
Table 2. Collection of samples and culture results.
| Sample type | Samples collected from EMCH | Samples collected from GMCH | Total samples | Proportion of positive samples | ||
| Number of samples | Positive for P. aeruginosa | Number of samples | Positive for P. aeruginosa | |||
| Urine | 72 | 16 | 43 | 7 | 115 | 23 (20%) |
| Urinary Catheter | 7 | 4 | 5 | 1 | 12 | 5 (42%) |
| Sec. wound infection | 12 | 7 | 9 | 4 | 21 | 11 (52%) |
| Pus | 37 | 5 | 32 | 5 | 69 | 10 (14%) |
| Burn wound | 8 | 2 | 2 | 0 | 10 | 2 (20%) |
| Blood | 9 | 1 | 0 | 0 | 9 | 1 (11%) |
| Tracheal aspirate | 2 | 1 | 0 | 0 | 2 | 1 (50%) |
| Total | 147 | 36 | 91 | 17 | 238 | 53 (22%) |
All of the 16 imipenem-resistant P. aeruginosa isolates were subjected to enzymatic activity and PCR-based genotypic detection of MBL variants. Of 16 isolates, 14 (~88%) were positive for MBL activity in imipenem-EDTA double-disk synergistic test. PCR-based detection of MBL variants, bla-VIM, bla-NDM1, bla-IMP, and bla-SPM, showed that 10 out of the 16 (~63%) resistant isolates carried at least one variant. While bla-VIM was detected in 7 isolates (44%) and bla-NDM-1 was detected in 3 isolates (19.75%) (Fig. 1). No MBL variant was detected in other 6 isolates. Moreover, none of the isolates were multiple MBL genes carrier (Table 3).
3.4. Detection of Plasmids in MBL-Positive Isolates
Plasmid extraction and gel electrophoresis showed the presence of plasmids in 7 out of 16 (43%) MBL-positive isolates. Among 7 bla-VIM positive isolates, 4 carried a plasmid of 1.2 MDa, while the other 3 isolates did not carry any plamid (Fig. 2). In constrast all 3 of the bla-NDM1 positive P. aeruginosa isolates carried a plasmid of 0.5 MDa (Table 3).
3.5. ERIC-PCR and Cluster Analysis
ERIC-PCR was performed to determine possible epidemiological relationships among the clinical P. aeruginosa isolates (21). Our results revealed distinct patterns of DNA, indicating species diversity among carbapenem-resistant P. aeruginosa isolates. Highly heterogeneous results indicated that isolates did not share any significant genetic relatedness. Phylogenetic cluster analysis based on unweighted pair group method with arithmetic mean (UPGMA) exhibited the presence of three distinct clusters I-III (Fig. 3). Majority of XDR isolates belonged to cluster III, while clusters I and II were composed of MDR isolates.
Table 3. Antibiotic susceptibility and resistance determinants in imipenem-resistant clinical P. aeruginosa.
| Isolate ID | Specimen (Hospital) |
MIC value (µg/ml) | Phenotypic detection of MBL production | Presence of MBL gene | Plasmid (MDa) | |||||
| IMP (Zone, mm) |
IMP+ EDTA (Zone, mm) | Interpretation | bla-IMP | bla-VIM | bla-SPM | bla-NDM1 | ||||
| PA01a | Reference | 0.5 | 19 | 20 | Negative | − | − | − | − | − |
| PU1 | Urine (A) | 8 | 0 | 0 | Negative | − | − | − | − | − |
| PU5 | Urine (A) | 8 | 0 | 14 | Negative | − | − | − | − | − |
| PU6 | Urine (A) | 8 | 0 | 15 | Intermediate | − | − | − | − | − |
| PUS1 | Cath. Swb (A) | 8 | 0 | 15 | Intermediate | − | − | − | − | − |
| PS1 | Wound Swb (A) | 16 | 0 | 16 | Intermediate | − | − | − | − | − |
| PS2 | Wound Swb (A) | 8 | 0 | 15 | Intermediate | − | − | − | − | − |
| PU17 | Urine (B) | 128 | 0 | 22 | Positive | − | − | − | + | 0.5 |
| PU26 | Urine (B) | 8 | 0 | 20 | Positive | − | + | − | − | 1.2 |
| PWS12 | Wound Swb (B) | 32 | 0 | 20 | Positive | − | + | − | − | − |
| PWS17 | Cath. Swb (B) | 128 | 0 | 24 | Positive | − | − | − | + | 0.5, 1.2 |
| PWS18 | Cath. Swb (B) | 128 | 0 | 21 | Positive | − | − | − | + | 0.5, 1.2 |
| PWS21 | Wound Swb (B) | 128 | 0 | 24 | Positive | − | + | − | − | 0.2, 1.2 |
| PWS22 | Wound Swb (B) | 16 | 0 | 22 | Positive | − | + | − | − | 0.2, 1.2 |
| PWS23 | Wound Swb (B) | 16 | 0 | 16 | Intermediate | − | + | − | − | − |
| PWS25 | Cath. Swb (B) | 8 | 0 | 16 | Intermediate | − | + | − | − | 0.2, 1.2 |
| PWS26 | Tracheal Asp (B) | 128 | 0 | 23 | Positive | − | + | − | − | − |
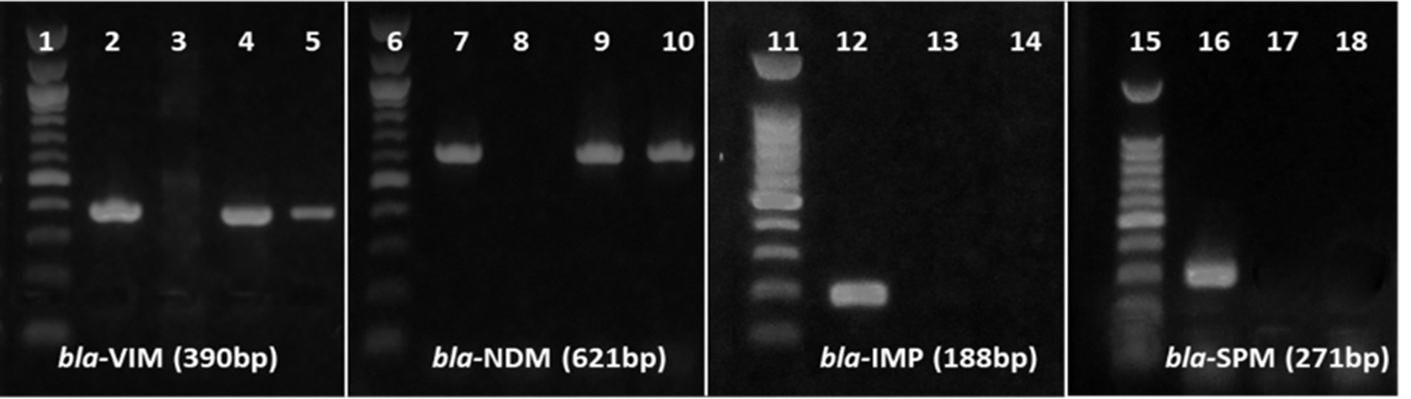
Figure 1. PCR-based detection of MBL variants. MBL gene sequences were PCR amplified using variant-specific primers. PCR products were electrophoresed through 1% agarose gel, stained with ethidium bromide, and visualized with UV light. The 100 bp DNA ladder indicates the size of PCR products. Lanes 2, 7, 12 and 16 represent variant-specific positive controls, and lanes 3, 8, 13 and 17 represent negative controls. Lanes 4, 5, 9, 10, 14 and 18 represent test isolates. No isolates were positive in bla-IMP and bla-SPM specific PCR. No isolates were positive in bla-IMP and bla-SPM specific PCR.
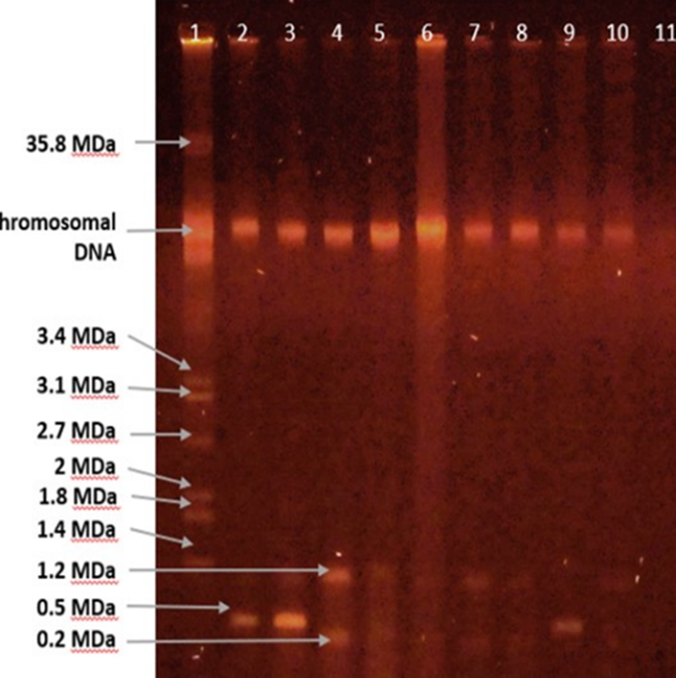
Figure 2. Plasmids in MBL-positive isolates. Plasmid extraction was conducted using modified hot alkaline lysis method. Plasmids were electrophoresed on 0.7% agarose gel stained with ethidium bromide, and visualized with UV light. Lane 1 represents the reference strain of E. coli K-12 V517 as visual marker; Lanes 2-11 represent extracted products from MBL-positive P. aeruginosa isolates.
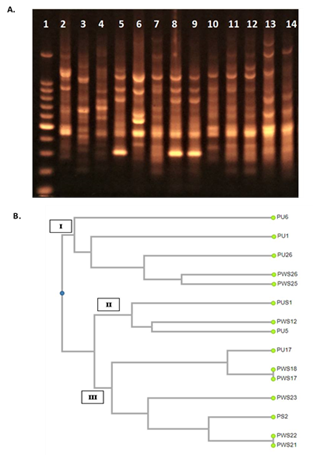
Figure 3. UPGMA cluster analysis of ERIC-PCR results. A). ERIC-PCR products were electrophoresed on 1% agarose gel, stained with ethidium bromide, and visualized with UV light. Lane 1 represents 100 bp DNA ladder; Lanes 8 and 9 represent isolates PWS18 and PWS17, respectively showing identical amplification pattern. Similarly, Lanes 10 and 11 represent isolates PWS22 and PWS21, respectively showing identical amplification. The rest of the lanes represent unique PCR amplification profiles. B). Dendrogram based on UPGMA cluster analysis of ERIC-PCR results; the names of the analyzed strains are mentioned with their phylogenetic groups denominated as Cluster I, II and III.
Rapid development of antibiotic-resistance in Gram-negative bacteria including P. aeruginosa has become a major public health concern all over the world (2, 3). There is an alarming increase in MDR, XDR, and pan drug resistance (PDR) isolates of P. aeruginosa (37, 38). In this study, we also observed frequent occurances of MDR among P. aeruginosa isolates in Bangladesh. Remarkably, one in every three isolates was resistant to carbapenems. While the interquartile range (IQR) of imipenem resistance reported in a systematic review covering studies during 2004 to 2018 was 13.5%, we detected a huge increase to 30% (6). Worldwide studies also state increasing occurrence of carbapenem-resistance in P. aeruginosa, including reports from China (77%), Serbia (43%), India (20%), and Japan (16%) (39-42).
Our results indicate that rapid increase in imipenem resistance among P. aeruginosa clinical isolates in Bangladesh is mediated by MBLs. About 88% of the resistant isolates were positive for MBL production. The MBL-positive isolates also showed an elevated level of MIC values of imipenem and meropenem. Resistance to carbapenems is often associated with acquisition and expression of the MBLs, which includes bla-IMP, bla-VIM, bla-GIM, bla-SPM, bla-NDM, and bla-SIM genes (29).
In this study 71% of carbapenem-resistant isolates were found to carry variants of MBL genes. While the majority (7/16; 44%) of the isolates carried bla-VIM, only 19% (3/16) carried bla-NDM-1. The current investigation shows much higher prevalence of MBL genes among P. aeruginosa isolates compared to similar studies conducted in India (12%), Pakistan (18%), China (55%), Iran (40%), and Nigeria (17%)(43-46). Moreover, it needs to be noted that we investigated the presence of only four variants (bla-IMP, bla-VIM, bla-SPM, and bla-NDM-1). Therefore, we cannot exclude the presence of other MBL variants in remaining 29% of the isolates.
In this study, bla-VIM variant was found to be the most prevalent among the MBL genes tested (7/16, 44%). Dissemination of novel variants of bla-VIM is commonly mediated by class I integrons like In58 and In59 gene cassettes (47, 48). Plasmids carrying class I integrons possess strong dissemination potential (49, 50). However, several of the bla-VIM carrier isolates (3/7, 42%) in our study did not carry any plasmid, implying that these isolates may have integrons in their chromosomal DNA. Although bla-IMP is globally the most predominant variant of MBL (51), none of the isolates harbored it. Rather, bla-VIM was found as the most prevalent variant in this study. This reflects the recent trend of frequent finding of carbapenem-resistant P. aeruginosa harboring bla-VIM, as reported by several international studies (39, 52).
In this study, the presence of bla-NDM-1 was detected among 3 of 16 carbapenem-resistant isolates. Classically bla-NDM-1 encoding gene is also plasmid-borne, which has been found to be disseminated through the members of the Enterobacteriaceae and Pseudomonaceae families (50, 53). Transmission of bla-NDM-1 has been also reported in Acinetobacter baumanii by transposon Tn125 (54, 55). It has been shown that bla-NDM-1 gene can be carried on plasmids of diverse sizes with incompatibility types that allow interspecies, intergenus, and interfamily transfer (56). In this study, we observed the presence of a 0.5 MDa plasmid in P. aeruginosa isolates that carried bla-NDM-1 gene. Remarkably, all bla-NDM-1 carriers were isolated from UTI and urinary catheter-associated infections.
We employed ERIC-PCR for molecular typing of genetic relatedness among P. aeruginosa isolates. ERIC-PCR revealed that the carbapenem-resistant P. aeruginosa isolates were distributed in three distinct clusters, with the majority belonging to cluster III. Overall analysis of our ERIC-PCR data indicated a heterogeneous nature of P. aeruginosa isolates and possible independent sources of dissemination. Several previous studies also reported similar heterogenous nature of P. aeruginosa isolates(44, 57). However, XDR isolates found in this study were all grouped in cluster III in ERIC-PCR analysis.
Our test samples were collected from two academic hospitals. Interestingly, both carbapenem resistance and presence of MBL pattern was different between two hospitals. Although 35% (6/17) of the P. aeruginosa from GMCH were carbapenem resistant, 2 were phenotypically MBL-negative and none were positive for the presence of MBL variants tested. In contrast, 28% (10/36) of the P. aeruginosa from EMCH were carbapenem resistant, but all the isolates carried MBL genes. Compared to GMCH, EMCH is a larger, tertiary care hospital that admits more patients for a longer period, which might establish a favorable niche for dissemination of MBL genes among nosocomial agents like P. aeruginosa. Previous studies also suggested that larger number of patients may harbor frequent source of drug resistant bacterial infections and prolonged hospitalization may increase the risk of acquisition of drug resistance elements through horizontal gene transfer (58, 59). A recent study in Bangladesh also demonstrated that hospital environment is a major reservoir for carbapenem-resistant P. aeruginosa with MBL carriage (60).
The major challenge of combating MBL-mediated carbapenem resistance lies with the rapid global dissemination of MBL through mobile genetic elements, their structural diversity, and discovering a broad-spectrum MBL-inhibitor without toxicity (61). Although the combination of ceftazidime-avibactam and aztreonam was found effective against MBL-producing Enterobacterales, they are less effective against P. aeruginosa for active intrinsic resistance (62). Newer antibiotics like cefiderocol also struggle from rapidly emerging resistance, thus, alternative treatment strategies to carbapenem-resistant P. aeruginosa infection now depends mostly on discovery of MBL inhibitors (61, 62). Inhibitors like taniborbactam show some promising aspect in neutralizing clinically relevant MBLs, but development of an inhibitor with broad-spectrum activity, metalloenzyme selectivity, metal-binding pharmacophore, and appropriate interaction in vivo is still an obstacle yet to overcome (63).
Indiscriminate use of therapeutically important antibiotics is presumed to be the major cause of antibiotic resistance and transmission of such resistant pathogens is more likely among hospitalized patients, especially those who need prolonged stay. Horizontal transfer of antibiotic resistance determinants further complicates the control strategies. Our results suggest that rapid screening by molecular techniques like ERIC-PCR may help track multidrug-resistant P. aeruginosa outbreaks in hospitals and develop point-of-care surveillance.
The emerging threat of ever-increasing antibiotic resistance among P. aeruginosa of clinical origin is a major concern for the treatment of nosocomial infections caused by the pathogens. The magnitude of MDR and the presence of MBL variants in clinical settings reported in this study require immediate attention to improve infection control and prevention programs. This study also suspects the hospital environment to facilitate the horizontal transfer of resistance elements like MBL genes. Implementation of antibiotic stewardship policy alongside proper infection control now has become a public health emergency to combat the emerging threat of superbugs in healthcare settings of developing countries like Bangladesh.
The authors would like to thank the laboratory personnel of Enam Medical College Hospital and Gonoshasthaya Medical College Hospital, Dhaka, Bangladesh, for their support in collection of the clinical samples.
Ethical Considerations
This study was approved by the Ethics and Research Review Committee of the Jahangirnagar University, Faculty of Biological Sciences [Ref No: BBEC, JU/M 2020 (1)4]. Written informed consent was obtained from patients and their personal identities along with other information were anonymized.
Authors’ Contributions
All authors made a significant contribution to the work reported. M.H.R. conceptualized and supervised the study. H.A., S.Y.M., M.S.A, and M.J.M. collected the clinical samples and conducted laboratory experiments. H.A. and S.Y.M. also analyzed the data and prepared the manuscript. M.S.H. and S.I. made significant contributions to the study design and M.A.K.R. edited the manuscript. The authors agree and approve the content of the final version of the manuscript.
M.H.R. received funding support from the Grants for Advanced Research in Education (GARE), the Ministry of Education, Bangladesh Award ID: (LS-2018661/Ref:bs-37.20.0000.004.033.020.2016.673) (website : https://moedu.gov.bd).
Conflicts of Interest
Received: 2024/08/1 | Accepted: 2024/12/31 | ePublished: 2025/01/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





