BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1451-en.html
2- Molecular Biology Research Center, Systems Biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran ,
3- Department of Microbiology, College of Basic Sciences, Shahr-e-Qods Branch, Islamic Azad University, Tehran, Iran
Increasing resistance of Klebsiella pneumoniae to antibiotics has made it one of the three pathogens threatening global health. Klebsiella is responsible for more than 10% of nosocomial infections (1). K. pneumoniae is a gram-negative pathogen belonging to the Enterobacteriaceae family, which causes several serious infections such as liver abscess, pneumonia, bacteremia and urinary tract infection (UTI) (2-4). K. pneumoniae is the most critical nosocomial infection due to its high mortality rate (5).
Klebsiella pneumoniae has two pathotypes, Hypervirulent K. pneumoniae (hvkp) and classical K. pneumoniae (Ckp), which differ in phenotypic and genotypic chara-cteristics (6, 7). Classical K. pneumoniae is the first pathotype to cause most infections. Hyperviolants are a type of Klebsiella pathotype with several bioma-rkers, including peg-344 (virulence of pulmonary infection), iroB, iucA (central nervous system invasion) (8, 9), and rmpA-rmpA2, and macA (increased prod-uction of antiphagocytic capsules) (7, 10). The Ckp and hvkp pathotypes are challenging to treat due to their antimicrobial resistance genes (7).
Klebsiella pneumoniae is a Superbug bacterium as it produces Extended-spectrum beta-lactamases (ESBL), carbapenemases, mobilized colistin resistance (mcr-1), and resistance to a large number of antibiotics (MDR-XDR) (11, 12). These bacteria pose many challenges in treatment (13, 14). Tetracyclines are currently widely used in livestock and humans due to their low toxicity, broad-spectrum activity against beta-lactamase-producing K. pneumoniae, tolera-bility, and easy market access (15, 16).
Unfortunately, the indiscriminate use of these antibiotics has led to antibiotic resistance. Tetrac-ycline resistance is caused by three mechanisms. First, overexpression of efflux pumps AcrAB-TolC and OqxAB, which reduces the cell's permeability to antibiotics due to the performance of efflux pumps and antibiotic resistance. Efflux genes are present in gram-positive and gram-negative bacteria (17). Second, ribosomal protection proteins, which protect ribosomes (S30 and S16) from tetracycline, alter the structure of these proteins, causing resistance to doxycycline and minocycline. Third, enzymatic chan-ges in antibiotics also cause resistance. The tetX gene causes antibiotic resistance due to tetracycline enzyme inactivation (18, 19). Currently, 23 genes encode the efflux pump and 11 genes encode it with ribosomal protection proteins (20, 21).
Therefore, due to the development of multidrug resistance to antibiotics in K. pneumoniae and various mutation mechanisms, the study of the pattern of antibiotic resistance leads to the appropriate admini-stration of antibiotics and faster recovery of related infections. The aim of this study was to investigate the phenotypic and genotypic resistance of tetracyclines in K. pneumoniae isolates in patients with urinary tract infections (UTTI) in Tehran hospitals, Iran.
Isolation and Identification of Isolates
In this study, 100 isolates of K. pneumoniae were isolated from clinical samples (urine) (2018-2019) from a major hospital in Tehran, Iran. Isolates were identified using conventional phenotypic and bioche-mical methods (22).
Antibiotic Susceptibility Testing
After identifying K. pneumoniae and culturing them, the antibiotic resistance pattern of K. pneum-oniae isolates was performed by standard disk diffu-sion method (Kirby-Bauer) according to CLSI (2016) instructions. Antibiotic resistance was determined for cefotaxime (CTX, 30g), ceftazidime (CAZ, 30g), erythr-omycin (E, 15g), and tetracycline (TET, 30g) (Padtan Teb - Isfahan). The results of antibiotic susceptibility testing of the samples after 24 hours of incubation at 37°C according to the standard table were evaluated based on the diameter of the stunted state, and the samples were classified into 3 groups: sensitive (S), semi-sensitive (I) and resistant (R) (22, 23).
DNA Extraction
The bacteria were cultured in LB medium at 37°C. After examining the turbidity of the tubes at 600 nm, bacterial DNA was extracted by a modified boiling method using STET buffer (Tris-Hcl 10 mM, NaCl 0.1 mM, EDTA 1 mM, pH = 8, Triton X -100) (Merck-Germany), the bacteria were extracted. Finally, the quality and quantity of genome concentration were evaluated by spectro-photometer at 260/260 nm. The extracted genome was stored at -20°C (24).
Molecular Study of tet39, tetB, tetC ٫ tetA Genes
After DNA extraction, tetracycline genes were analyzed by multiplex PCR. PCR reaction was perfor-med in a total volume of 25 µL containing 12 µL of Mastermix, 10 µL of distilled water, 0.5 µL of each specific primer (Table 1) (Pishgam Company - Tehran), and 1 µL of sample DNA. PCR conditions for gene amplification were designed based on the binding temperature of the primers (Table 2). Thermocycler (Bio-Rad, USA) was used for PCR reaction. PCR products were analyzed by electrophoresis on 2% agarose gel containing a safe stain (Pishgam - Tehran), and the presence of tetracycline genes in K. pneumoniae isolates was determined.
Table 1. The sequence of primers used for multiplex PCR of tetracycline genes, PCR product size, and binding temperature
| Reference | Connection temperature | PCR product size | Primers' sequence | Primer |
| (25) | 56 °C | 956bp | GTAATTCTGAGCACTGTCGC | tetAF |
| (26) | 56 °C | 956bp | CTGCCTGGACAACATTGCTT | tetAR |
| (26) | 56 °C | 415bp | CTCAGTATTCCAAGCCTTTG | tetBF |
| (26) | 56 °C | 415bp | ACTCCCCTGAGCTTGAGGGG | tetBR |
| (26) | 56 °C | 505bp | CCTCTTGCGGGATATCGTCC | tetCF |
| (26) | 56 °C | 505bp | GGTTGAAGGCTCTCAAGGGC | tetCR |
| (26) | 56 °C | 701bp | CTCCTTCTCTATTGTGGCTA | tet39F |
| (26) | 56 °C | 701bp | CACTAATACCTCTGGACATCA | tet39R |
Table 2. Thermal program used for genes used for PCR
| 95°C | 1 Cycle | 5 min | Denaturation | |||||
| 95°C | 30 Cycle | 30 s | Denaturation | |||||
| 56°C | 30 Cycle | 30 s | Annealing | |||||
| 72°C | 30 Cycle | 30 s | Extension | |||||
| 72°C | 1 Cycle | 5 min | Final extension |
Out of 100 clinical samples (urine), 54 samples were collected from male subjects and 46 samples from females, and their age range was between 30 and 80. According to the CLSI table (2016), there were 42 tetracycline-resistant samples, 12 cefotaxime-resis-tant isolates, 15 ceftazidime-resistant isolates, and 83 erythromycin-resistant isolates (Figure 1). According to the PCR multiplex test results, tetracycline resistance is high in K. pneumoniae isolates. So that 31 samples were positive for tetA gene, 8 samples for tetB gene, 21 samples for tetC gene, and 8 isolates for tet39 gene. Also, 5 samples were positive for tetA and tetB genes, 4 isolates for tet39 and tetA genes, and 14 samples for tetA and tetC genes. Most samples were positive for the tetA gene. No isolate tested positive for all four genes simultaneously (Figures 1, 2 and 3).

Figure 1. Percentage of antibiotic resistance and susceptibility in K. pneumoniae
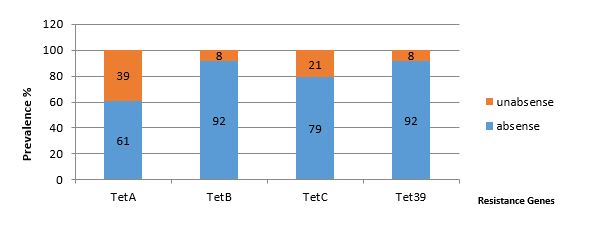
Figure 2. Percentage of tetracycline resistance genes in K. pneumoniae
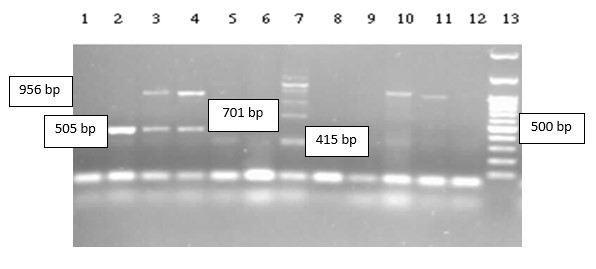
Figure 3. Multiplex electrophoresis PCR of tetracycline (tet) genes, product sizes are tetB = 415bp, tetC = 505 bp, tet39 = 701bp, tetA = 956 bp. Well 13: Molecular marker (100bp), band size from bottom to top -100 bp - 200 bp - 300 bp -400 bp -500 bp (sharp band) - 600 bp - 700 bp - 800 bp - 900 bp -1000bp
Klebsiella pneumoniae is a gram-negative intestinal bacterium that forms part of the natural flora of the human body. This bacterium causes a wide range of diseases, including bacteremia, pneumonia, UTI, liver abscesses, and sepsis. In recent decades, due to the indiscriminate and unscientific use of antibiotics, we have witnessed the emergence and spread of drug-resistant strains in this bacterium. In 2014, the World Health Organization (WHO) reported K. pneumoniae as one of three antibiotic-resistant pathogens. The global spread of drug-resistant strains is a serious threat to global health (23, 27).
Among the antibiotics studied in this study, erythromycin and tetracycline demonstrated the highest resistance, with ceftazidime and cefotaxime the lowest resistance. According to Heidari et al. (2018), the resistance of K. pneumoniae to ceftazidime ratio was 55.7%. Even though this antibiotic is a powerful tool against K. pneumoniae, the resistance to ceftazidime is high. This difference can be due to the indiscriminate use of antibiotics in different geographical areas (28).
Klebsiella pneumoniae is the most common species of Klebsiella, which causes human infections. By prod-ucing beta-lactamase, it causes hydrolysis and inacti-vation of most beta-lactam antibiotics such as penicill-ins, cephalosporins and monobactams. But it does not affect cephamycin and carbapenem. Carbapenems were among the antibiotics used to treat drug-resistant infections in the past. But today, they are a health threat due to the production of carbapen-emases by Enterobacteriaceae (26).
Our results showed that out of 100 isolates of K. pneumoniae, 12 samples were resistant to cefotaxime and 15 to ceftazidime. While a 2016 study by Ribeiro et al. on 75 samples of Klebsiella showed that all isolates were resistant to ceftazidime and cefotaxime (29). In the present study, cefotaxime and ceftazidime were reported to be effective antibiotics. The results of these two studies were inconsistent, which could be due to the indiscriminate use of these antibiotics and the emergence of new resistant strains or the acquisition and transfer of plasmid resistance genes . Multiplex PCR methods for detecting tet resistance genes have not been investigated so far, and this is the first research in this regard. In 2017, Taitt et al. performed phenotypic and genotypic antibiotic resistance tests on 87 K. pneumoniae specimens. The results showed that more than 2.3 of the isolates are resistant to 5 or more antibiotics. This is a threat to public health and must be considered a critical challenge by the Centers for Disease Control and Prevention (30).
Most isolates were resistant to tetracycline, which was consistent with the recent study that reported 44% tetracycline resistance, which may be due to the proximity of these isolates in these two regions. The results obtained from the frequency of tetracycline genes in the present study are slightly consistent with the study of this group. The study reported the frequency of tetA and tetB genes to be 16% and 9%, respectively (31). While in the present study, the frequency of tetA genes is 39%, and tetB is 8%. The prevalence of the tetA gene has been reported to be higher, which may be due to mutations in the gene, but the prevalence of the tetB gene is almost equal and consistent.
The study by Bokaeian et al. (2014) was conducted on 30 samples of K. pneumoniae. The results of this group showed that the isolates were resistant to erythromycin (70%), cefixime (53%), tetracycline (50%), and ceftazidime (36%). All samples were positive for tetA and tetB genes. In the present study, all samples were resistant to erythromycin (93%), tetracycline (42%), cefotaxime (12%), and ceftazidime (15%). Moreover, 39 samples tested positive for the tetA gene and 8 isolates for the tetB gene. In both studies, erythromycin was reported to be the most resistant antibiotic in this respect. But in other cases, more than our results, it was reported that this discre-pancy is due to incorrect administration of antibiotics in the treatment of infections or transfer of resistance genes by various transport agents such as integrons, plasmid R, transposons, and bacteriophages (32).
Another study was conducted by Kashefieh et al. (2019) on 100 samples of K. pneumoniae. The freq-uency of 42%, 30%, 16%, and 21% were reported for tetB, tetA, tetC, tetD, respectively. The prevalence of the tetA gene is consistent with the present study, but the frequency of other genes has been reported to be more. This discrepancy may be due to the indiscr-iminate use of antibiotics in different geographical areas (33).
Antibiotic resistance genes are found in gram-negative and gram-positive bacteria. In the Adelowo study, which reported 13 microorganisms from contaminated water, tetA, B, and C were not observed, and 8 isolates (three gram-positive and five gram-negative bacteria) were positive for tet39. In a recent study, 8 isolates tested positive for the tet39 gene. Therefore, the two studies are consistent.
The absence of other genes in the isolates may be due to limited samples. Tetracycline resistance is the most common type of antibiotic resistance that requires monitoring of tet39 resistance gene diversity (34). Adding antibiotics (tetracycline, erythromycin, streptomycin, and beta-lactamases) to animal feed is considered a source of antibiotic resistance that poses challenges in treating human and animal infections (35).
The results of this study reported resistance of K. pneumoniae isolates to erythromycin and tetracycline antibiotics. Most samples were positive for 2 tetracycline resistance genes, and none of the 4 resistance genes were observed simultaneously.
Thank to guidance and advice from the ClinicalResearch Development Unit of Baqiyatallah Hospital,Tehran, Iran.
his study was funded by the authors.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2021/08/26 | Accepted: 2021/12/22 | ePublished: 2022/02/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








