BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1300-en.html
2- Department of Biology, Faculty of Basic Sciences, Islamic Azad University, Roudehen Branch, Tehran, Iran ,
The skin is the largest organ and the outer covering of the body of living organisms. Any damage such as wounding to this protective organ exposes the living organism to severe risks. Wounding activates the healing process, which includes different phases regulated by multiple growth factors and cytokines released from the wound site (1,2). Various treatment methods have been proposed to treat wounds, including treatment with natural compounds or chemical drugs. However, the investigation and identification of probiotic bacteria have directed the attention of scientific forums toward probiotic therapy.
Probiotic is derived from the Latin preposition “pro”, meaning for, and the Greek word “biotic”, meaning life (3). This term refers to living organisms with health effects on the host body if consumed in sufficient quantities. Lilly and Stillwell first used the term probiotic in 1965, but Élie Metchnikoff, a Russian immunologist, studied the therapeutic effects of bacteria in his book, ‘The Prolongation of Life’ in 1907 (4). Since then, extensive research has been conducted on the therapeutic effects and function of probiotics. In vitro and in vivo experiments in humans and animal models have shown that probiotics can regulate the immune system in infectious, allergic, and inflammatory conditions. The most important and common probiotics belong to the genera Lactobacillus and Bifidobacterium. Valdez et al. pointed to the effective use of probiotic lactobacilli to treat gastrointestinal disorders, indicating their ability to secrete acids, bacteriocins, and other by-products that can neutralize infections resulting from pathogens (5). Satish et al. referred to the anti-pathogenic effects of probiotic bacteria previously described by other researchers and stated that their study was the first characterization of probiotic therapy, consistent with reduced scarring (6). A study by Lolou et al. at the University of Newcastle showed that the use of Lactobacillus and Bifidobacterium could reduce skin inflammation and affect the treatment of atopic dermatitis and allergic contact dermatitis (7). Another study by Walaa Mohammedsaeed (2015) at the University of Manchester investigated the therapeutic effect of probiotic Lactobacillus rhamnosus on skin wounds through in vivo cell culture and in vitro skin culture. Lactobacillus rhamnosus stimulated the re-epithelialization process in both models by activating cell proliferation and migration. According to the results of this study, the therapeutic effect of Lactobacillus rhamnosus on cell migration and keratinocyte proliferation may lead to upregulation of cxcl2/cxcr2 expression. These findings suggest that the cxcl2 gene contributes significantly to the wound healing process by stimulating keratinocyte migration, proliferation, and binding (8). Mirani et al. (2017) developed a multifunctional dressing for wound management in their study at Victoria University. This engineered hydrogel-based dressing called GelDerm kept the wound site moist and prevented the growth of pathogenic microorganisms (9).
Although the therapeutic effects of probiotics, particularly Lactobacillus, on gastric ulcers and skin wounds have been studied at the national and international levels, little research has been conducted on the effect of Bifidobacterium spp., which has both known similarities and differences with Lactobacillus spp., on the healing of skin wounds. Thus, the present study took this novel approach into account and investigated the therapeutic effect of the Bifidobacterium bifidum probiotic bacterium by the application of bacterial culture supernatant in acute wound healing in female (BALB/c) mice in the form of the novel dressing design. On the other hand, Aloe vera hydrogel was used in this dressing to keep the wound moist, alleviate pain by cooling, and control side effects such as fissures in the wound site, which cause fragility and further damage.
2.1 Chemicals and Reagents
All the chemical reagents for preparing hydrogel and MRS (de Man, Rogosa, and Sharpe) culture to prepare supernatant are purchased from (Merck kGaA, Darmstadt, Germany). Ketamine and Xylazine (Alfasan Diergeneesmiddelen B.V, Netherland) were used to anesthetize mice. Live culture of Bifidobacterium bifidum (PTCC: 1644) was obtained from the Iranian Research Organization for Science and Technology (IROST), Tehran, Iran, in 2020.
2.2 Preparation of Bifidobacterium bifidum Supernatant
Live culture of B. bifidum was plated in MRS broth (pH value 5.5), co-cultured at 37°C for 72 h, under microaerophilic condition to reach microbial suspension (3  108 CFU.mL-1) (10). Prepared suspension incubated at 4°C for 15 min, and centrifuged at 1700
108 CFU.mL-1) (10). Prepared suspension incubated at 4°C for 15 min, and centrifuged at 1700  . The supernatant was separated and filtered through a 0.2
. The supernatant was separated and filtered through a 0.2  m microfilter.
m microfilter.
2.3 Preparation of Hydrogel
0.1 gr of Methyl Paraben and 100 ml of water with Aloe Vera gel were placed in Bain-marie at 50-60°C for 20-30 min. The mixture was passed through the Whatman Filter Paper. 100 mL of water and 10 gr of Carbopol were added, and the mixture was completely homogeneous. Finally, 4 gr of Glycerol was added and stored in the refrigerator for 24 h.
2.4 Induction of Skin Wound on Mice and treatment with drug formulation
In this study, 44 Female BALB/c mice (6-8 week-old) weighting (approximately 18-23 gr) were obtained from the Pasteur Institute, Tehran, Iran. In order to adapt to the new environment, mice were housed for one week in the Animal laboratory of the Islamic Azad University, Central Tehran Branch, in separate and sterile cages. During the study, the temperature was 22  , 12 h day and night cycle with full access to water and food were considered. All the principles of keeping laboratory animals were in accordance with the ethical guidelines of the university (IR.IAU.SRB.REC.1398.118). These animals were then randomly divided into experimental, sham, controls, and healthy groups. To induce injury, mice were anesthetized by intraperitoneal injection of the Ketamine 10 mg/kg and Xylazine 20 mg/kg.
, 12 h day and night cycle with full access to water and food were considered. All the principles of keeping laboratory animals were in accordance with the ethical guidelines of the university (IR.IAU.SRB.REC.1398.118). These animals were then randomly divided into experimental, sham, controls, and healthy groups. To induce injury, mice were anesthetized by intraperitoneal injection of the Ketamine 10 mg/kg and Xylazine 20 mg/kg.
After shaving the dorsal surface, 8 mm acute wound was made using biopsy punches (Kai, Japan) (11). Then the whole skin injury site was excised, and the surgery day was considered as the 0 day. Through the phases, the ethical principles were respected and prevented from any physical harm. Mice were divided into five groups considered as experimental 1, experimental 2, sham, negative and positive controls. There were five mice in experimental 1 and 2, positive and negative control groups, and there were three mice in the sham group. Also, three mice were grouped as the healthy group. Every 48 h treatments were administrated for each mouse; 50 ml of 72 h bacterial culture supernatant with 8 mL of hydrogel prepared as a drug base for experimental group1 and Eucerin for experimental 2 were placed on sterile gas and dressed. The negative control group was untreated. Positive control only dressed with Eucerin. In the sham group, hydrogel was used for dressing. After all, wound sizes were measured by caliper every 48 hours (12).
2.5 Histological Studies
Mice were euthanized on days 7 and 14; biopsy sites were excised, fixed in 10% formalin, and processed for routine histology. The section was stained with haematoxylin – eosin (measuring the wound diameter and counting the blood vessels) photographed with a bright-field microscope (Olympus, Tokyo, Japan) (13-15).
2.6 Statistical Analysis
The results were analyzed using SPSS software version 26 (SPSS Inc., Chicago, IL., USA) by one-way (ANOVA) and (Tukey) test. Values were given as the mean standard error of the mean. The P- values are shown as P-value< 0.05.
Histological Studies
3.1 Measuring Wound Diameter in the Study Groups
As can be seen in figure 1, wound diameters in all groups showed a significant decrease on days 7 and 14 of the study compared to day 0 (8000 µm). On the 7th day of this study, wound diameter was found in the negative control group 4111.10± 82.72 and in the positive control and sham were found 3306.48±34.79 and 3024.77±60.70 µm, respectively. The wound diameters in experimental groups 1 and 2, on day 7th were measured 870.67±75.30 and 2132.99±78.57, respectively. The results of the 14th day were obtained from the negative, positive and sham equivalent to 2159.97±146.09, 1373.69±85.53, and 935.50±72.17. In the case of experimental groups, wound diameters were measured *124.23±56.2 and *415.08±33.04 (P<0.05).
Figure 1. Wound diameter changes in different groups during the treatment period. Results are expressed as mean  standard error of the mean. The P- values were considered significant at *P< 0.05 levels.
standard error of the mean. The P- values were considered significant at *P< 0.05 levels.
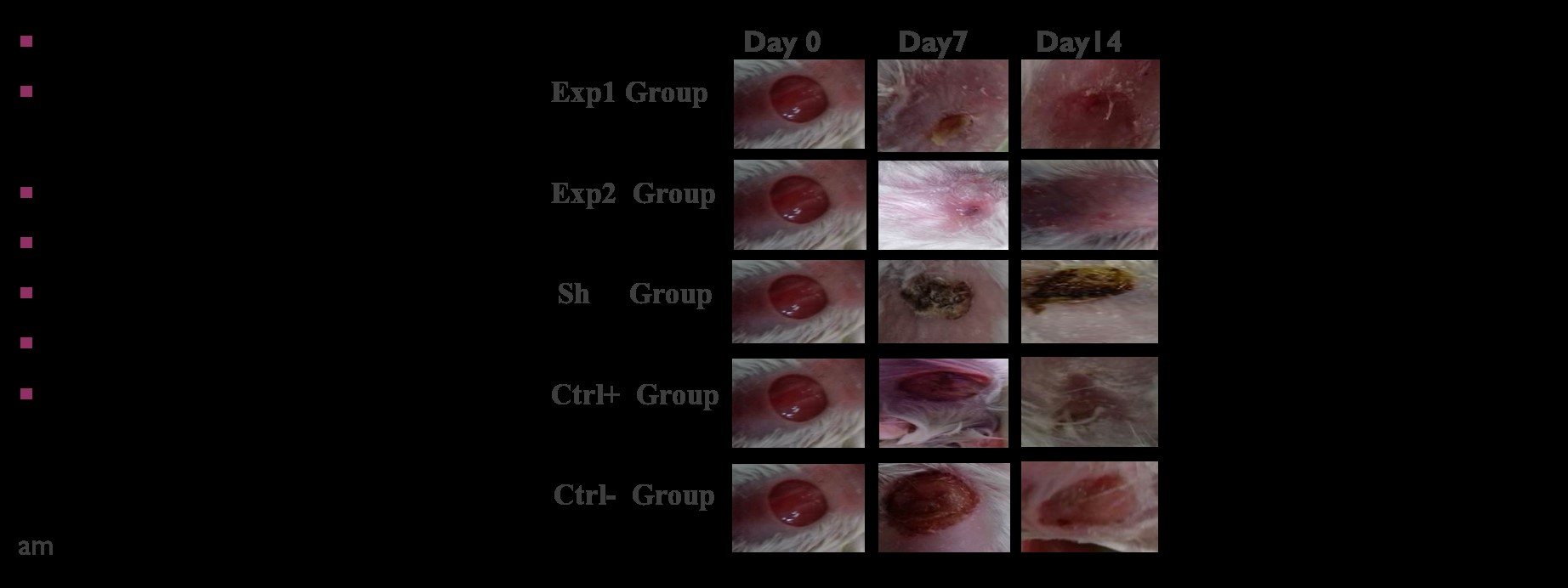
Figure 2. Macroscopic images of wound samples under different treatments at days 0, 7, and 14 of the study.
3.2 Counting blood vessels (Angiogenesis indicator)
Figure 3 shows the rate of angiogenesis indicator (in 500 µm2) during the wound healing process in different groups on the study days. The obtained results in this study show that the rate of angiogenesis on the 7th day of the study was 2.41±0.08, 4.25±0.72, and 9.33±0.30 micrometers in the negative control, positive control, and sham groups, respectively. On 14th day of study, they changed to 4.75±0.90, 5.83±0.36, and 6.91±0.36, respectively. In case of experimental groups 1 and 2, compared to 0 day, the rate of angiogenesis on the 7th day of the study increased from 11.33±0.46 to14.33±0.33; Also angiogenesis decreased to 11.50±0.38 and 9.08±0.33 on 14th day of the study (P<0.05).
Figure 3. Changes in the number of blood vessels in different groups during the treatment process. The highest number of blood vessels belonged to experimental group1. Results are expressed as mean  standard error of the mean. The P- values were considered significant at *P< 0.05 levels.
standard error of the mean. The P- values were considered significant at *P< 0.05 levels.
Figure 4. Microscopic images of the wound section. In the study groups during 14 days of study. Tissue samples were stained with hematoxylin- eosin and tissue images were photographed with Olympus microscope on days 0, 7, and 14 of the study. Yellow arrows in groups indicate blood vessels. The highest number of blood vessels was seen on day 7 in the experimental group1. A section of different parts of skin belongs to experimental group 1, numbered on the 14 day of study is in the following figure: 1. Healthy skin, 2. Wound location, 3. Epidermis, 4. Dermis, 5. Blood vessel, 6. Hair follicle, 7. Sebaceous glands
This study aimed to investigate the effect of probiotic bacteria on the duration of acute wound healing. Hence, an experiment was designed to determine the most effective indicator of acute wound healing by measuring various qualitative indicators according to the known mechanisms of wound healing. Regarding numerous studies previously conducted on lactobacilli in healing gastrointestinal ulcers and scarce research on the role of bifidobacteria in healing skin wounds, the study focused on the potential role of Bifidobacterium bifidum probiotic bacteria to reduce the duration of the treatment. Previous studies have employed probiotic bacteria in various forms, such as extracts and cell lysates (8, 16). Given the possible toxic and fatal effects of living probiotic bacteria in the blood, the topical application of supernatant resulting from bacterial cell culturing could be more appropriate in terms of stability at room temperature compared to live cells, making it suitable for in vitro and in vivo studies (8, 17). This experiment would rather use bacterial culture supernatant instead of living bacteria. A study by Walaa Mohammedsaeed (2015) at the University of Manchester also showed that lysed Lactobacillus rhamnosus G.G did not have harmful effects as the live bacteria. Moreover, lysed probiotics are more cost-effective for the treatment of skin infections, as there is no need to preserve living bacteria (8).
The in vitro experiment of the current study examined 44 female BALB/c mice in six groups. The results of this experiment were analyzed concerning the indicators of wound diameter and number of blood vessels, assessed by macroscopic studies and calculated by histological studies along with statistical analysis, respectively. Reduction in the wound size and depth is a determining indicator during the healing period. This important repair response begins in the proliferative phase with the migration of fibroblasts and myofibroblasts to the wound. Proliferation and production of matrix and procollagen proteins 1 and 3 at the end of the first post-injury week and extracellular matrix density later support cell migration and the repair process. Wound contraction occurs with the removal of fibroblasts. In the remodeling phase, collagen is regenerated, wounds are completely consolidated, and collagen fibers are intertwined to reduce the scar thickness (18, 19).
The present study showed that the application of the probiotic Bifidobacterium bifidum led to the reduction of treatment duration. The data obtained in accordance with cellular and molecular mechanisms of wound healing showed the most significant decrease in the indicator of wound diameter in the experimental groups compared to sham, positive, and negative control groups (P <0.05) (Figure 1). A comparison of the experimental groups 1 and 2 indicated more and faster healing response of wound contraction in experimental group 1 (P<0.05) with the smaller wound size on the seventh day of the treatment. The probiotic agent seemed to shorten the duration of the inflammatory phase, leading to faster onset of fibroblasts migration and proliferation phase. Previous studies have mentioned the role of Toll-like receptors (TLRs) expression in neutrophils, fibroblasts, monocytes, and macrophages in the repair process. Researchers believe that these may be due to probiotic function through TLRs that play a key role in the innate immune system (20).
Angiogenesis and vasculogenesis play a crucial role in wound healing. Neovascularization or angiogenesis is involved in the growth of new capillaries to form granular tissue. Three to five days after tissue damage, the capillaries become visible as granular tissue forms on the wound site, functioning as a network for blood vessel proliferation, fibroblast migration, and new collagen (21,22). Any factor that can accelerate the formation of blood vessels in the wound and establish blood circulation to the tissue area helps to stimulate the wound healing process and prevent the wound deepening. In this study, the culture supernatant of the Bifidobacterium bifidum showed the potential to increase angiogenesis in the experimental groups. Hence, a comparison of the experimental group 1 and the sham group showed that despite the common hydrogel composition in both dressings and the antibacterial and antiviral properties of the constituents of hydrogels (23,24) in preventing microbial growth and reducing inflammation and pain (8,25), the experimental group 1 has a better performance in the process of wound healing and angiogenesis because of the probiotic agent.
As the results show, experimental groups 1 and 2 had the highest angiogenesis on the 7th day, respectively, compared to the control groups. This result suggests that the increase in angiogenesis is due to the probiotic bacterium Bifidobacterium bifidum function. It is well known that the rate of angiogenesis in granular tissue reaches its peak around the 7th day of injury, after which the blood vessels are gradually destroyed. In this experiment, the highest rate of angiogenesis occurred on the 7th day of treatments in experimental group 1, which is consistent with previous studies (P<0.05). Given the ascending trend of angiogenesis until the 7th day of the injury, such as what happens in the proliferative phase, and its gradual decline, the quantitative index of blood vessels follows a decreasing trend from the 7th to 14th day of the study in the experimental groups, which is consistent with re-epithelialization phase (Figure 2) (P<0.05). The use of bacterial culture supernatant also seems to act as a stimulant and increase angiogenesis (Figure 2). Previous studies have shown that it is possible to improve angiogenesis by activated macrophages and epidermal tissue. Until recently, acid or basal fibroblast growth factors seemed to be responsible for such activities, but it is now clear that other molecules such as vascular endothelial growth factor (VEGF), angiogenin, and angiopoietin are involved in angiogenic activities (19). Keratinocytes produce VEGF from the early to the final stages of wound healing. Activated fibroblasts, mast cells, and macrophages express VEGF in injured skin. In fact, myeloid cells (monocytes and macrophages) are the major source of VEGF in the post-injury stages. Studies have also shown the effects of both keratinocyte-derived and myeloid cell-derived VEGF on wound healing (26). It also seems that the tissue stimulates macrophages to secrete VEGF in the face of hypoxia (8). Studies on mouse models have shown that a lack of VEGF in myeloid cells and keratinocytes delays wound contraction, decreases blood vessel density, and reduces granular tissue formation. Low levels of oxygen in injured skin activate the Hypoxia Inducible Factor, leading to VEGF gene transcription (26). A study in mice showed that probiotics increased the expression of VEGF and TGF-β (27).
As shown by Halper et al., Lactobacillus extract stimulates the production of TNF-α and angiogenesis in the inflammatory phase of tissue repair as inflammatory cells such as macrophages, lymphocytes, and plasma cells support pro-inflammatory processes (28).
According to the intragroup comparison of experimental groups 1 and 2 in the present study, Eucerin did not contribute to wound healing and the increasing number of blood vessels. There are no reports on the antibacterial or antiviral properties of Eucerin, which softens and relieves dry skin. Therefore, a careful comparison of the groups showed that although the bacterial culture supernatant coupled with Eucerin affected the wound healing process, the use of this liquid with hydrogel had the greatest effect on wound healing and angiogenesis (P<0.05). No infection was observed in the experimental groups of the present study as probiotics prevented wound infection by antimicrobial mechanisms, including secretion of antimicrobial peptides, inhibition of bacterial invasion, and inhibition of pathogenic bacteria adhesion to epithelial cells (29). On the other hand, the intra-group comparisons of the control groups also showed no significant differences in the wound diameter and the number of blood vessels in the group receiving no treatment (negative control) and the group receiving Eucerin dressing (positive control).
In general, as the results of this study showed, increased angiogenesis and decreased wound diameter was consistent with previous studies (5, 6), while the probiotic bacterium Bifidobacterium bifidum reduced the duration of treatment. It is well known that time plays a crucial role in reducing or increasing the outcomes of wounding (30, 31). Given the significant decrease in the wound diameter and increase in angiogenesis of the experimental groups on the 7th day, it seems that shorter phases of three, five, seven, and nine days (32) may help determine a more accurate interval for these two indicators in future studies. It is also possible to use the bacterial culture supernatant of Bifidobacterium bifidum probiotic bacterium alone and investigate its effects.
According to the results of the current study, the probiotic bacterium Bifidobacterium bifidum reduces wound diameter, increases angiogenesis, and decreases the duration of the treatment significantly (P<0.05). This study, which is in line with research on the therapeutic effects of probiotics in scientific and academic centers of Iran and other parts of the world (8), indicates the effectiveness and usefulness of this therapeutic method. Besides, this study shows that the bacterial culture supernatant of Bifidobacterium bifidum probiotic bacterium can be an alternative and reliable treatment. Thus, it is possible to use the designed biological dressing containing bacterial culture supernatant of Bifidobacterium bifidum probiotic bacterium and Aloe vera hydrogel to treat acute wounds after further studies.
This current research article has been adapted from the dissertation of Ms. Pegah Moussavi Amin with University code number 33262. The ethical instructions were approved by the Vice-Chancellor for Research and Technology of the Islamic Azad University, East Tehran Branch. Also, thanks go to everyone who has helped us to achieve this important goal.
Author Disclosure Statement
The authors have no conflict of interest concerning this manuscript.
Funding
This article is an independent study that was conducted without organizational financial support.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2021/03/13 | Accepted: 2021/08/13 | ePublished: 2021/09/5
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |











