BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1208-en.html

 , Ashrafalsadat Hatamian-Zarmi2
, Ashrafalsadat Hatamian-Zarmi2 
 , Bahman Ebrahimi Hosseinzadeh1
, Bahman Ebrahimi Hosseinzadeh1 
 , Zahra-Beagom Mokhtari-Hosseini3
, Zahra-Beagom Mokhtari-Hosseini3 

2- Department of Life Science Engineering, Faculty of New Sciences and Technologies, University of Tehran, Tehran, Iran ,
3- Department of Chemical Engineering, Faculty of Petroleum and Petrochemical Engineering, Hakim Sabzevari University, Sabzevar, Iran
.
The history of using medicinal fungi in East Asian countries dates back to thousands of years ago. Many of these fungi are still used to treat diseases (1). The medicinal fungi properties include stimulating the proliferation of lymphocytes, reducing the proliferation of cancer cells, and anti-inflammation. The increasing importance of medicinal fungi and their metabolites in the treatment of various diseases, the appropriate biodiversity of medicinal fungi in Iran, and the economic value of metabolites derived from these fungi indicate the need to pay attention to this branch of biological sciences (2). Among the medicinal fungi, Basidiomycete Fomes fomentarius has long been used in the treatment of gastrointestinal diseases, liver cirrhosis, and various cancers. The distribution of this fungus is widespread in Iran and its presence has been reported in Mazandaran, Golestan, Gilan, Isfahan, Tehran, Kurdistan, Kermanshah, Khorasan and Azerbaijan provinces (2, 3). This fungus has important properties in various aspects. This species is considered as one of the important causes of white heart rot on forest trees. Its extract has antioxidant and anti-cancer properties. F. fomentarius is also considered for the production of the laccase enzyme and use in processes such as decolorization and biodegradation (3-5). One of the bioactive compounds of this fungus is polysaccharides that have anti-cancer, anti-inflammatory, anti-diabetic, and immune-enhancing activities. F. fomentarius β-glucan polysaccharide prevents tumor angiogenesis and metastasis (6, 7). The anti-proliferative effect of this polysaccharide has been observed on SGC-7901, A549, MCF7, and MKN-45 cancer cell lines (4, 5, 8). Studies show that the polysaccharides of F. fomentarius also have antibacterial and antiviral activity (9, 10). Chen et al. (2008), examined the biomass and polysaccharide production of F. fomentarius in submerging culture conditions. A temperature of 25°C and an initial pH in the range of 5-6 are suitable for the growth of this fungus. Also, using glucose as carbon source, yeast extract as a nitrogen source, CaCl2 and MgSO4.7H2O improves mycelial growth and increases polysaccharide production (11). Cultures of F. fomentarius in stirred bioreactor and solid bed bioreactor have also been studied (4, 12). Many studies have been done on optimizing the culture medium for the growth of other fungi and their metabolite production. One of these statistical methods is the Taguchi array design, which allows the study and optimization of several variables simultaneously (13). Therefore, in this study, the production of biomass and polysaccharide of the Iranian medicinal fungus F. fomentarius, which has been isolated from the forests of Mazandaran, was investigated. Also, the composition of the culture medium of this fungus was optimized to increase the production of biomass and polysaccharide by the Taguchi method. Then the biological activities of polysaccharides including antibacterial, anti-oxidant, and cytotoxicity were investigated.
Collection of Mushroom Samples
The F. fomentarius was isolated from Mazandaran forests in 2017 with the cooperation of Sari Agricultural Sciences and Natural Resources University. In each case, the fungal specimen was harvested in a healthy, complete and appropriate manner based on the color, shape, size and surface decoration of the cap, base, blades and many other characteristics. After morphological confirmation by mycologists, it is kept at 4°C.
Cultivation of F. fomentarius and Polysaccharides Extraction
The F. fomentarius mycelium was cultured in potato dextrose agar (PDA) (manufactured by Merck, Germany) in a petri dish and incubated for 5 days at 28°C (BINDER, USA). For seed culture, 10 mm2 pieces of fungus grown in PDA medium were transferred to 100 mL of Potato dextrose broth (PDB) medium (manufactured by Merck, Germany) for 7 days at 28°C and 150 rpm in shaker incubator (JAL TAJHIZ, Iran). Suitable culture medium of this fungus includes glucose (5%), peptone (0.2%), malt extract (1%), yeast extract (0.2%), MgSO4.7H2O (0.25%) and KH2PO4 (0.5%). The initial pH of this medium was adjusted to 6 by adding NaOH (1M) and HCl (1M). After autoclaving, 5% v/v inoculum was added and incubated at 28°C and 150 rpm for 4 days (11). To extract the F. fomentarius polysaccharide, after separating the biomass using Whatman paper, absolute ethanol with a ratio of 4:1 v/v was added to the supernatant. After overnight refrigeration, the polysaccharide precipitates were centrifuged for 10 min and 10,000 rpm (AWEL/MF20-R/France). The biomass and polysaccharide were lyophilized (OPERON) and the final product was stored at room temperature.
Optimization of Culture Medium Compositions by Taguchi Method
Taguchi method was used to investigate the effect of independent variables of MgSO4.7H2O, pH, yeast extract, and inoculum percentage on biomass and polysaccharide production. The Taguchi L9 array was used to examine four variables at three levels (Table 1), the experiments were performed according to this array, and the results were analyzed using Design Expert 11 software (State-Ease, USA).
Biological Assessments
Antibacterial Activity
The colony-forming unit (CFU) method was used to evaluate the antibacterial activity of polysaccharides. Staphylococcus aureus (UTMC 1429) and Escherichia coli (PTCC 1269) were obtained from the the Research Center for Technology and Microbial Products of the University of Tehran and Persian Type Culture Collection. Bacteria were cultured in Müller-Hinton Broth (manufactured by Merck, Germany) and after 24 hours of incubation (BINDER, USA), 0.5 McFarland solution was prepared from them. A 5% solution of each polysaccharide with 0.5 McFarland solution was prepared from each bacterium in a volume of 500 μL and kept for 24 hours in a shaker incubator (JAL TAJHIZ, Iran) at 37°C and 140 rpm. The mixture was diluted with phosphate buffer saline (1:10) and 10 μL of it cultured in nutrient agar medium (manufactured by Merck, Germany). In the control state, 10 μL of McFarland 0.5 solution of each bacteria was cultured. Then, all culture media were incubated and after 24 hours, bacterial colony count was performed with a gel dock (Quantum, France) (14).
Antioxidant Activity
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) test was used to evaluate the antioxidant activity of polysaccharides. After preparing 2 mg/mL polysaccharide solution in water, the polysaccharide solution and DPPH solution were mixed in a ratio of 1:4 v/v and placed in a shaker incubator for 30 minutes (10). Then the absorbance of the samples was measured at 517 nm with ELISA reader (Carry 100 Bio, Australia) and antioxidant activity was calculated according to the following formula:
:(eq.1)
DPPH• scavenging effect (%) = [(A0 – sample A)/A0] × 100
A0: Adsorption of DPPH solution at 517 nm
sample A: Absorption of the test sample at 517 nm
Cytotoxicity Assay
MKN45, AGS, A549, KYSE-30, and 5637 cancer cell lines were purchased from the Iranian Genetic Resources Center. Cancer cells were cultured in RPMI 1640 medium with 2 mM L-glutamine and 15% FBS and stored in a CO2 incubator at 37°C. To evaluate the effect of polysaccharide, its solution was prepared in sterile water (1 mg/mL) and used at specific concentrations (50, 100, 200 µg/mL) to treat cells for 24 and 72 hours. In this experiment, positive control included culture medium and negative control included untreated cell culture. MTS test was used to evaluate cell viability. A total of 2000 cancer cells were cultured in each well of 96 well plates (24 hours). After treatment with specified concentrations of polysaccharide for 24 and 72 hours, 10 µM MTS solution (1:10 diluted in RPMI 1640) was added to each well and incubated for 3 hours (15). Then, adsorption of each sample was read at 495 nm using ELISA reader (Carry 100 Bio, Australia) and the cell viability percentage was calculated using the following formula:
:(eq.2)
Cell viability: Sample absorbance/ Control absorbance × 100
Statistical Analysis
Data were analyzed statistically by ANOVA method using Design Expert, version 11 (State Ease, USA). P-values below 0.05 (P<0.05) were considered to be statistically significant.
Optimization of Biomass and Polysaccharide Production
According to the preliminary results, the optimization of the culture medium was designed and performed using the Taguchi method with four factors at three levels (Table 1).
| Parameters | Response | |||||
| Runs | A:MgSO4.7H2O (g/L) | B:pH | C:Yeast extract (g/L) | D:Inoculum (% v/v) | Biomass (g/L) | Polysaccharide (g/L) |
| 1 | 2.5 | 8 | 2 | 5 | 9.890 | 4.498 |
| 2 | 2.5 | 4 | 4 | 10 | 10.560 | 2.337 |
| 3 | 1 | 4 | 2 | 3 | 11.840 | 1.274 |
| 4 | 1 | 6 | 4 | 5 | 14.522 | 2.785 |
| 5 | 4 | 4 | 6 | 5 | 11.626 | 4.201 |
| 6 | 1 | 8 | 6 | 10 | 9.920 | 2.878 |
| 7 | 2.5 | 6 | 6 | 3 | 14.690 | 3.573 |
| 8 | 4 | 8 | 4 | 3 | 12.150 | 5.410 |
| 9 | 4 | 6 | 2 | 10 | 7.600 | 3.110 |
:(eq.3)
Biomass: 10.44 - 1.44A – 0.4200B + 1.55C - 3.18D
In this equation (A) is the concentration of MgSO4.7H2O, (B) pH, (C) yeast extract, and (D) the percentage of inoculum. The two-dimensional and three-dimensional diagrams of the effect of variables on biomass production are shown in Figure1.
| Source | Sum of squares | df | Mean square | F-value | P-value |
| Model | 41.69 | 4 | 10.42 | 9.57 | 0.0251 |
| MgSO4.7H2O (A) | 5.55 | 1 | 5.55 | 5.55 | 0.0868 |
| pH (B) | 1.06 | 1 | 1.06 | 0.9722 | 0.3800 |
| Yeast extract (C) | 6.41 | 1 | 6.41 | 5.88 | 0.0723 |
| Inoculum (D) | 28.67 | 1 | 28.67 | 26.34 | 0.0068 |
| Residual | 4.35 | 4 | 1.09 | ||
| Cor Total | 46.05 | 8 |
:(eq.4)
Polysaccharide=3.71+1.45A+0.8292B+0.4427C-0.6025D
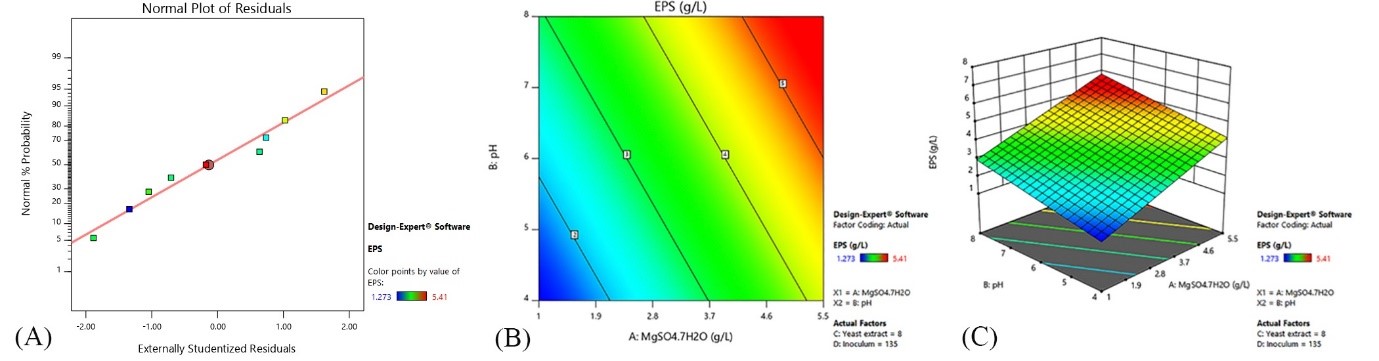
In this equation (A) is the concentration of MgSO4.7H2O, (B) pH, (C) yeast extract and (D) the percentage of inoculum. The two-dimensional and three-dimensional diagrams of the effect of variables on polysaccharide production are shown in Figure 2.
| Source | Sum of squares | df | Mean square | F-value | P-value |
| Model | 11.25 | 4 | 2.81 | 11.02 | 0.0196 |
| MgSO4.7H2O (A) | 5.58 | 1 | 5.58 | 21.84 | 0.0095 |
| pH (B) | 4.13 | 1 | 4.13 | 16.16 | 0.0159 |
| Yeast extract (C) | 0.5227 | 1 | 0.5227 | 2.05 | 0.2257 |
| Inoculum (D) | 1.03 | 1 | 1.03 | 4.02 | 0.1153 |
| Residual | 1.02 | 4 | 0.2553 | ||
| Cor Total | 12.27 | 8 |
Antibacterial Activity
The antibacterial activity of polysaccharide produced by F. fomentarius in Taguchi experiments was tested against Gram-positive bacteria S. aureus and Gram-negative bacteria E. coli. Bacterial colony count using a gel dock device showed that in the control sample of both strains, bacterial growth is 100%. Colony counts showed that the polysaccharide of this fungus has an inhibitory effect on bacterial growth. This effect is greater on the gram-positive bacteria S. aureus. Sample 7 with 50% inhibition of S. aureus had the highest inhibitory effect (P<0.05). It can also inhibit the growth of E. coli by up to 24%. The highest inhibitory effect on E. coli was observed in sample 3 (25%). There was no significant difference between the antibacterial activities of polysaccharides on E. coli. The Figure 3 (A) shows the antibacterial activity of the polysaccharide of the fungus F. fomentarius.
Antioxidant Activity
The antioxidant activity of polysaccharides produced by F. fomentarius in Taguchi experiments was investigated using DPPH free radicals. When the antioxidant substance donates protons to this free radical, its absorption in 517 nm decreases; this decrease indicates the level of antioxidant activity. The antioxidant activity of polysaccharides produced by this fungus varies in different environments (Figure 3, B). Analysis of DPPH results showed that the antioxidant activity in polysaccharide produced in environment 7 shows the highest antioxidant activity (16.11%). The lowest antioxidant activity is related to sample 2.
Cytotoxicity Assay
Biological assessments in previous stages showed that sample 7 has the most biological activity; therefore, this sample was used to evaluate cytotoxicity. In this study, the antiproliferative effect of polysaccharide of this fungus in different concentrations on 5 cancer cell lines MKN-45, AGS, A549, KYSE-30 and 5637 was investigated using MTS test (Figure 4). In general, F. fomentarius polysaccharide inhibits the growth of all studied cancer cells significantly (P<0.05). After 24 hours, the antiproliferative effect of polysaccharide at a concentration of 50 µg/mL on AGS was significantly (P<0.05) greater than that KYSE-30 and 5637, but no difference was observed between the antiproliferative effect of polysaccharide in other cancer cell lines. At a concentration of 100 µg/mL polysaccharide had the highest antiproliferative effect on the AGS cell line and it was significantly higher than other cell lines (P<0.05). At a concentration of 200 µg/mL, the polysaccharide has a significant antiproliferative effect on A549 cancer cells compared to other cell lines, and it inhibits the growth of this cell by more than 50%. After 72 hours, there was no significant difference between the antiproliferative effects of polysaccharide on the cancer cell lines. At a concentration of 100 µg/mL polysaccharide of F. fomentarius had the most antiproliferative effect on 5637 cells and it was significantly different from other cell lines (P<0.05). At a concentration of 200 µg/mL, polysaccharide inhibits the growth of A549, KYSE-30 and 5637 cells by up to 40% and has a significant difference compared to this effect on MKN-45 and AGS cells (P<0.05).

Figure 3. Antibacterial (A) and antioxidant (B) activity of polysaccharide obtained from the Taguchi L9 array.
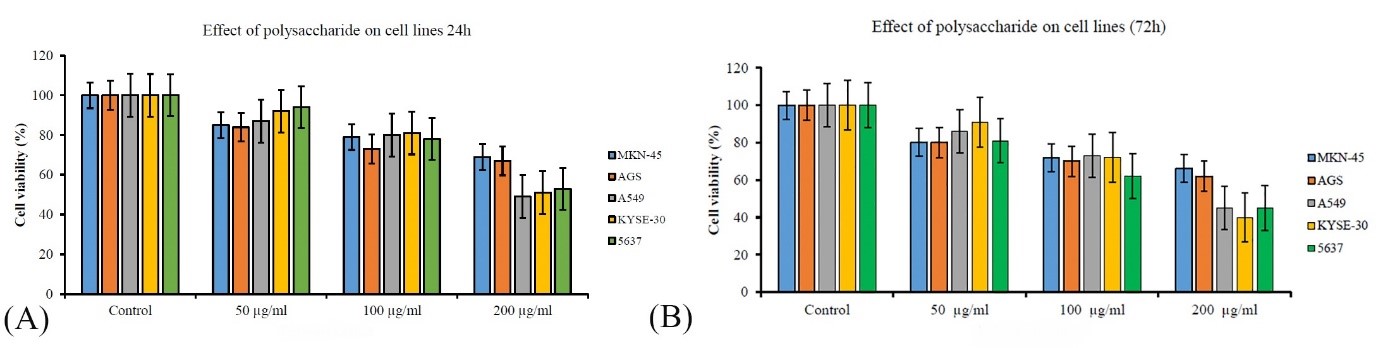
Figure 4. Cell viability diagram of 5 cancer cell lines treated with F. fomentarius polysaccharide after 24 (A) and 72 hours (B)
In this study, the optimization of biomass and polysaccharide production of the medicinal fungus F. fomentarius and its polysaccharide biological properties were investigated. As observed in previous studies (4) and this study, the suitable culture medium for this fungus includes glucose, peptone, malt extract, yeast extract, MgSO4.7H2O, and KH2PO4. Studies show that peptone in this environment plays an important role in increasing the growth and production of fungal enzymes (16). Optimization of culture medium using the Taguchi method showed that MgSO4.7H2O concentration and initial pH have a significant effect (P<0.05) on polysaccharide production of this fungus. With the increase of MgSO4.7H2O and the initial pH, the production of polysaccharide of F. fomentarius increases. pH is involved in permeability, cell membrane function, and production of secondary metabolites (11). Magnesium is an important element in the metabolism of fungi and the stability of cell membranes. It is also involved in DNA replication, cell division, and many enzymatic reactions (11, 17, 18). Chen et al. )2008(, increased the polysaccharide production of this fungus (3.64 g/L) by optimizing the culture medium. In this study, under optimal conditions (4 g/L MgSO4.7H2O, initial pH=8), the production of polysaccharide of the F. fomentarius reached 5.410 g/L. This amount is 1.5 times the production of polysaccharides in common fungal culture medium (PDB) (11).
Studies show that physicochemical conditions of the culture medium such as carbon source, nitrogen source, mineral sources, pH and culture temperature play an important role in the biological activity of fungal metabolites (19, 20). F. fomentarius polysaccharide inhibits the growth of S. aureus and E. coli by 50% and 25%, respectively. Polysaccharide of this fungus contains terpenoids and polyphenols with antibacterial activity and with their increase this activity increases (21). Studies show that the type and volume of solvent used to extract polysaccharides play a role in the content of polyphenols. The use of polar solvents such as ethanol increases polyphenols (10, 22). Kalyoncu et al. (2010), found that the antioxidant activity of the polysaccharide of the F. fomentarius was 5.97%. By optimizing the culture conditions in this study, the antioxidant activity of polysaccharide increased to 16.11%. Most biological properties such as antioxidant, antibacterial, and antiproliferative activity are observed when the initial pH of the fungal culture medium is in the range of 5-7 (23). The difference between the initial pH and the composition of the culture medium causes a difference in the phenolic content of the polysaccharide. These compounds are associated with antioxidant activity and reduction of OH•, O2•, and NO• radicals (24). Investigations in this study and previous studies (4, 5, 11) show that this polysaccharide has an antiproliferative effect on cancer cells, but this effect varies in cell lines and increases with increasing of the concentration. The antiproliferative effect of F. fomentarius polysaccharide was observed (KYSE-30> A549 563749> AGS> MKN-45) after 72 hours. In the treatment of KYSE-30 cells with 200 µg/mL polysaccharide, cell viability reaches 40% after 72 hours. Also, after 72 hours, the life expectancy of A549 and 5637 cancer cells was 45% and that of MKN-45 and AGS cancer cells was 66% and 62%, respectively. Today, the polysaccharides of the fungi like Ganoderma lucidum and Tinea versicolor are used as supplements in the treatment of various cancers. The anti-cancer activity of these polysaccharides includes inhibiting the growth of cancer cells and stimulating the immune system (25). The antiproliferative activity of fungal polysaccharides is related to the chemical structure and composition of their monosaccharides. The polysaccharide of F. fomentarius is rich in beta-glucan, which has anti-cancer properties. This compound destroys transcription proteins in cancer cells and arrests the cell cycle in the G1 phase. It also induces apoptosis in cells by damaging organelles, the cell nucleus, and fragmenting DNA (10, 26, 27, 28). The results of this study showed that the polysaccharide of the medicinal F. fomentarius, due to its antibacterial, antioxidant and antiproliferative properties, can be a suitable option for the treatment of many diseases and can be used as a dietary supplement.
In this study, the production of biomass and polysaccharide of the Iranian medicinal F. fomentarius was optimized using the Taguchi method. Inoculum percentage had significant effects on biomass production and MgSO4.7H2O concentration and initial pH had significant effects (P<0.05) on polysaccharide production of this fungus and under optimal conditions (4 g/L MgSO4.7H2O, initial pH=8) polysaccharide production increases 1.5 times and reaches 5.410 g/L. Biological assessments showed that this polysaccharide inhibits the growth of S. aureus and E. coli 50% and 25%, respectively (P<0.05). Also, its antioxidant activity increases 2 times and reaches 16.11%. The antiproliferative effect of F. fomentarius polysaccharide is different on different cancer cells and increases with increasing concentration. The cell viability of KYSE-30 treatment with 200 µg/mL polysaccharide, reaches 40% after 72 hours. Also, the cell viability of A549 and 5637 cancer cells is 45% and the cell viability of MKN-45 and AGS cancer cells is 66% and 62%, respectively.
This article is taken from the master's thesis (19/06/28690), which would like to thank all the professors who helped us in the Faculty of New Sciences and Technologies, University of Tehran.
Funding for this study was provided by the authors in collaboration with the University of Tehran.
Authors declared no conflict of interests.
Received: 2020/08/27 | Accepted: 2020/10/5 | ePublished: 2020/10/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |