BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1125-en.html
2- Department of Microbiology and Virology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
3- Chemistry Department, Payam-e Noor University, Mashhad, Iran ,
.
The emergence of antibiotic resistance in bacteria is one of the most important global health issues, and new research is needed around the world to develop more effective antimicrobial compounds (1). In the 21st century, nanotechnology has become one of the most important and influential technologies in science worldwide. Nanomaterial engineering can open up new avenues of research and development in some fields including medicine, cosmetics, agriculture, environmental sciences, materials science, biomedical sciences, information, and food technology. It is also used to understand the changes in the physicochemical properties of nanomaterials (5). In fact, nanotechnology covers a wide range of sciences. In developing countries, it is important to invest and focus on this science to make improvements in the fields of health, water, energy, and environment (6,7). Nanomaterials are structures that have at least one dimension on the nanometer scale (1-100 nanometers). On a broad spectrum, nanoparticles have shown impacts against both gram-positive and gram-negative bacteria (8). For example, zinc oxide nanoparticles could inhibit Staphylococcus aureus and silver nanoparticles have shown concentration-dependent antimicrobial activity against E. coli and Pseudomonas aeruginosa (9). Recently, nanoparticles (NPs) have drawn much attention because of their potential to be an alternative to antibiotics. The unique properties of nanoparticles compared to the balk form make them ideal for diagnosis, treatment, and antibiotic delivery systems. Rapid and sensitive detection of bacteria can be done by nanoparticle-based methods. In nanotechnology, there are new perspectives for the development of new formulations based on different types of nanoparticles with different sizes, shapes, and antimicrobial properties (11). Nanoparticles might seem to be a promising solution because not only can they fight against bacteria, but they can also act as carriers of antibiotics and natural antimicrobial compounds. This study focuses on the mechanisms of bacterial resistance and antibacterial activity of nanoparticles because it is very important to investigate the antibacterial mechanisms of these particles to produce more effective antimicrobial agents.
Standard requirements for literature reviews were conducted in the English language listed on health and medical electronic databases. The search words were: "Bacteria,” “Nanotechnology,” “Antibacterial,” and “Nano” using PubMed, Scopus, Science Direct, and Google Scholar databases. Furthermore, manual searches of other relevant journals and keywords searches were performed. We have focused on published papers from 2010 to2020. After the initial review, the following types of articles were excluded: (i) studies that were not relevant to the main subject, (ii) papers which their full texts were not available, and (iii) repetitive publications and articles with similar results. We included articles that discussed the following topics: Articles that have examined the antibacterial mechanisms of nanoparticles on bacteria, as well as articles that explored the benefits and application of nanoparticles to combat bacteria, and finally studies that examined factors that affect the antibacterial mechanisms of nanoparticles.
Mechanisms of Bacterial Resistance to Antibiotics
Bacterial resistance occurs due to the widespread use of antibiotics in prevention or treatment of diseases without proper medical symptoms, which has become a serious problem. This resistance has several reasons, all of which occurred due to the interaction of internal and external factors (12). External factors usually include antibiotics pressure and human-caused environmental changes, and internal factors include resistance from the genetic point of view and at the DNA level, as well as resistance from the biochemical point of view and at the protein level (14,15).
Advantages of using Nanoparticles in treating Bacterial Infections
Bacteria can develop resistance to antimicrobials. Nanoparticles can combat microbes through various mechanisms that are simultaneously activated. Simultaneous mechanisms greatly reduce the possibility of several mutations in different genes, and most antibiotic resistance mechanisms are irrelevant to nanoparticle resistance mechanism; therefore, it is hoped that bacteria will be less resistant to nanoparticles (17). Nanoparticles are widely affected by the immune system. Many studies have shown that they have the ability to strengthen the immune system. The produced nanoparticles enter the body as a foreign substance, in return, the innate immune system responds immediately to these substances. This response can vary depending on the physicochemical properties of nanoparticles, including the size, charge, shape, and degree of hydrophobicity. As a result, the interaction of nanoparticles with various components of the immune system such as neutrophils, soluble proteins, and antigen-processing cells (APCs) depends on nanoparticle properties (18). Another case of stimulation of the immune system by nanoparticles is the development of allergic reactions. There are also many immune system reactions triggered by nanoparticles, which are produced by the production of inflammatory cytokines. Numerous studies have reported the induction of cytokines with different types of nanomaterials (gold colloids, dendrimers, polymers, lipid nanoparticles, etc.). Based on the body's immune response to nanoparticles, these substances can be used to treat cancers and autoimmune diseases, and act as a vaccine (19-20). Information about the different effects of nanoparticles on cells of the innate immune system (macrophages, dendritic cells, neutrophils, mast cells, and natural killer cells) and cells of the adaptive immune system (T cells and B cells) helps us understand the immunological effects of nanomaterials. Innate immune system cells such as macrophages and dendritic cells cause the expression of inflammatory cytokines, activation of T cell lymphocytes and activation of inflammasome. In addition, Nanoparticles can secrete histamine and increase cytosolic calcium ions by affecting on mast cells. Also nanoparticles are able to target natural killer cells (NK cell) to control their movement toward the tumor. By affecting the B cells of the adaptive immune system, the nanoparticles enhance immunity during vaccination and help target B-cell lymphoma without the need for chemotherapy. The interaction of nanoparticles with T lymphocytes can lead to Th1 and Th2 responses as well as cytokine production and it helps development of nanoparticle-based products. Moreover, nanoparticles can act as a carrier for antibiotics synergistic factor and as a transvestite factor, which can ultimately facilitate therapeutic efficacy (21,22). The combination of nanoparticles (NPs) and antimicrobial materials, such as antibiotics, peptides, or different biomolecules, has been known as one of the most effective methods targeting antibiotic resistance. Due to their small size, they can be used to treat infections caused by intracellular pathogens (24,25). They also protect the drugs from the effects of enzymes and harmful chemical reactions. Nanoparticle carriers can help accurately target the infection site through antibiotics. Therefore, the unwanted side effects due to high doses and systemic side effects are minimized. With the use of these types of carriers, the drug concentration at the site of infection can reach the required effective level and be stable for a long time. Carrier nanoparticles can combine and transmit several antibacterial drugs together (26-29).
The Problems of using Nanoparticles
Bacterial Resistance to Nanoparticles
Further research has identified new aspects regarding the effects of nanoparticles on bacteria. While most previous studies have mentioned that nanoparticles, as an antibacterial agent, prevent the development of resistant bacteria, new information has recently been found to support the opposite. One study reported the increase in plasmid transport (such as RP4, PK2, and pCF10) by aluminum nanoparticles, which leads to the formation of resistant strains (30).
Toxicity
Local and systemic toxicity, as well as harmful effects on beneficial bacteria in the human body, are among the concerns discussed regarding the use of nanoparticles. Some NPs can cause hemolysis and interfere with blood coagulation pathways. One study has shown the deposition of silver nanoparticles in the liver, spleen, lungs, and other organs, which leads to organ damage. Intravenous nanoparticles may accumulate in the large intestine, lungs, bone marrow, liver, spleen, and lymphatic system, and their inhalation can cause toxicity in the lungs. Besides, several studies showed that nanoparticles do not have any dangerous toxicity in vivo conditions. The exact mechanism of the toxic effects of nanoparticles in laboratory conditions is unclear; therefore, further studies are required (31).
Application of Nanoparticles
Nanoparticles are used in different fields due to their unique properties and their effect on the bacteria. Different Types of nanoparticles have their characteristics, and each type is used for antibacterial purposes in certain parts of the body. Currently, there are some undergoing clinical trials on drugs with metal-based nanoparticles against bacterial infections with name Arikace, Lipoquin, Pulmaquin, Silvasorb, MAT2501 and QA-PEI. The cost of producing and using these nanoparticles is very high compared to traditional medicines; therefore, conventional treatments are more commonly used. Finally, nanoparticle-based drugs may be preferred in specific conditions and can increase the patients' quality of life (32).
Antibacterial Mechanisms of Nanoparticles
Despite special attention to nanomaterials, their antibacterial mechanisms have not been well known. But the accepted and important antibacterial mechanisms of nanoparticles against bacteria will be discussed here.
The Function of Nanoparticles due to Oxidative Stress
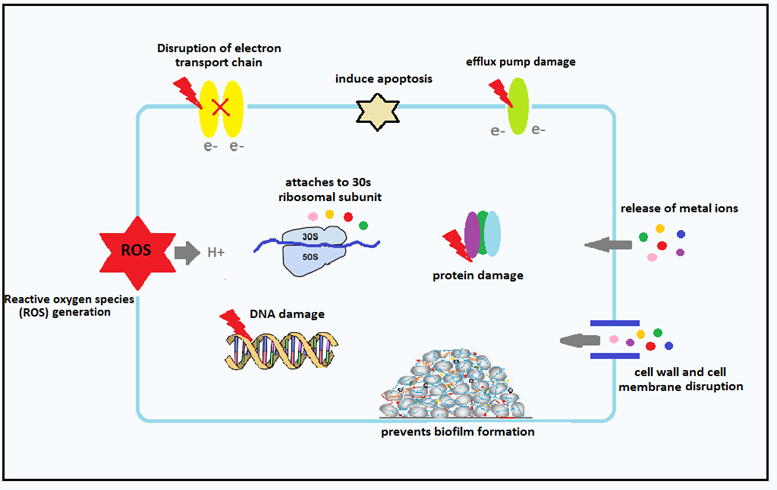
Oxidative stress induced by ROS is an important antibacterial mechanism of NPs. It can attack proteins and reduce the activity of some periplasmic enzymes. Moreover, reactive oxygen species are effective in increasing gene expression levels of oxidative proteins which are considered an important mechanism in bacterial cell apoptosis (33-34). It can destroy cellular components of the pathogens like membrane, DNA, and mitochondria, which can result in cell death (35). The study carried out by Ansari et al. confirmed that Al2O3 nanoparticles pass through the cell membrane and enter the cell, then nanoparticle-membrane interaction and due to intracellular oxidative stress, membrane integrity is damaged (36). Nanoparticles can also bind to TrxR and Trx active sites and interfere with the thioredoxin system, which is one of the most important redox systems of disulfide that is used by bacteria against oxidative stress, and leads to oligomerization and dysfunction of TrxR and TrxR (37).
Performance of Nanoparticles by Producing Soluble Metal Ions
The next important mechanism is releasing metal ions from metal oxide. Metal ions are slowly released from metal oxide and then absorbed through the cell membrane, followed by direct interaction with functional groups such as proteins and nucleic acids. This interaction alters cell structure, disrupts enzymatic activity, and interrupts the common physiological activity in bacterial cells (42).
Mechanisms of Nanoparticles Penetration into Bacteria
Diffusion: Nanoparticles can transmit reactive oxygen species to bacteria using diffusion. In a study, Pan et al. showed that iron oxide-graphene oxide nanoparticles could inactivate methicillin-resistant Staphylococcus aureus (MRSA) due to the production of large amounts of hydroxyl radicals and diffusion into bacterial cells (43).
Adsorption: Metal nanoparticles are released into the environment and bind to negatively charged groups in the bacterial cell membrane, such as carboxylic and phosphate groups, in a process known as biological adsorption. In a study, it was shown that the surface charges of copper nanoparticles significantly affect the adsorption of nanoparticles to membranes (46).

Figure 1. The nanoparticles application in medicine
Interaction of Nanoparticles with the Cell Membrane and Bacterial Cell Wall
Studies showed that the interaction of nanoparticles with cell membranes in gram-positive and gram-negative bacteria is different due to their specific structure. Lipopolysaccharide (LPS) is a unique structure of the cell wall of gram-negative bacteria that creates a negatively charged region and absorbs nanoparticles. In contrast, teichoic acid is only expressed in the cell wall of gram-positive bacteria; therefore, nanoparticles are distributed along with the phosphate molecular structure and prevent their accumulation. Many studies have shown that nanoparticles have more antibacterial activity against gram-positive bacteria in comparison with gram-negative bacteria because the cell wall of gram-negative bacteria is composed of lipopolysaccharides, lipoproteins, and phospholipids. Nanoparticles can transmit reactive species of oxygen into bacteria through diffusion (47). The results of a study by Lucanhan et al. showed that selenium nanoparticles have a strong electrostatic repulsion compared to lipopolysaccharide and gram-negative bacterial membranes due to their very negative nature. Gram-positive bacteria, on the other hand, have significantly less negative charge than gram-negative bacteria. Therefore, selenium nanoparticles are more likely to be deposited on the surface of gram-positive bacteria and cause bacterial death (39). Foster et al. confirmed that titanium oxide nanoparticles can adhere to the surface of bacterial cells to produce reactive oxygen species and damage the composition and structure of cell membranes, thereby they disrupt cell membrane function and cause cell leakage, and ultimately kill the bacteria (50). One of the most important functions of the cell membrane is the respiratory activity of bacteria. Studies have reported that nanoparticles also disrupt the respiratory activity of bacterial cell membranes (52). Figure 2 shows the attachment, penetration and cell lysis of bacteria with Kan-AuNPs treatment (83)
In recent years, the effect of nanoparticles in the synthesis of bacterial proteins has received much attention from researchers. Su et al. investigated the effect of copper nanoparticles on nitrification of bacteria by maQing a significant change in the expression of key proteins. After entering the cell, proteomic bioinformatics analysis showed that copper nanoparticles regulate proteins involved in nitrogen metabolism, electron transfer, and material transport (54). Iron oxide nanoparticles were studied based on their strong tendency to form disulfide bonds and it was shown that they affect the metabolism and redox status of bacterial cells.
Nanoparticles can also stop protein and DNA synthesis in bacteria by inhibiting the combination of ribosomal subunits with tRNA or restricting ATPase activity to reduce ATP levels (55-56). Studies have indicated that nanoparticles can bind to proteins in cell membranes and create stable bonds. And as a result, they inactivate proteins that are involved in the production of membrane ATP and mediate the transfer of ions through cell membranes (10).
Regulation of the Expression of Metabolic Genes by Nanoparticles
The expression of metabolic genes is regulated by nanoparticles. Nanoparticles can also regulate cell membrane penetration and interfere with molecular pathways. Bacterial metabolic processes play a major role in bacterial growth and reproduction. They can also cause pathogenicity in bacteria. Therefore, targeted alteration of bacterial metabolic activity can be used to regulate bacterial pathogenicity For example, it has been observed that copper oxide nanoparticles can regulate the expression of proteins involved in bacterial nitrogen metabolism and significantly inhibit the activity of nitrate reductase and nitrite reductase. Wang et al. reported that silver nanoparticles can be covalently bonded to thiol derivatives and attached to some genetic components without any complicated modification process (47-48).
Inhibition of Bacterial Biofilms Formation by Nanoparticles
Prevention of biofilm formation by nanoparticles is an important mechanism because biofilms play an important role in the development of bacterial resistance. Genotypic and phenotypic properties of cells in biofilms are different from those of free cells, and these differences make them highly resistant to antibiotics (35). By the use of silver nitrate (AgNO3), Mohanti was able to produce silver nanoparticles with proper anti-biofilm effects (60). Pan et al. also reported that nanoparticles could affect the metabolic rate of bacterial communities. Bacterial metabolism is considered an important activity for biofilms. Based on other studies, nanoparticles can prevent the formation of biofilm by preventing bonding and icaAD transcription. Smaller nanoparticles are more capable of destroying bacterial biofilms due to the increase in surface-to-mass ratio (61). Due to the small size of nanoparticles, they can penetrate microbial cell walls and biofilm layers and cause irreversible damages to cell membranes and DNA. The shape of nanoparticles affects the destruction of biofilms. for instance, rod-like nanoparticles are more effective at destroying biofilms than spherical nanoparticles (64).
| Mechanisms of antibacterial action | Targeted bacteria | nanoparticles |
|---|---|---|
| -Interference with electron and energy transfer through the membrane -Prevention of DNA replication and electron transport chain in bacteria and fungi -Disruption of the cell surface and loss of membrane integrity -Penetration into bacterial biofilms using an external magnetic field -Generation of reactive oxygen species (ROS) -Combination with antibiotics such as vancomycin, tetracycline and ampicillin |
Methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis. Vancomycin-resistant Enterococcus faecium and Klebsiella pneumoniae | Ag Nps |
| -Generation of reactive oxygen species (ROS), lipid peroxidation, alkaline, electrostatic interference | S. aureus, E. coli, Bacillus megaterium, Bacillus subtilis | MgO NPs |
| -Generation of reactive oxygen species (ROS), superoxide radicals -release of ion and binding to the thiol group in bacterial surface proteins -DNA damage |
S. aureus, E. coli | TiO2 NPs |
| -Generation of reactive oxygen species (ROS) -Penetration of hydrogen peroxide produced at the surface of nanoparticles into bacterial cells -Prevention of enzymes product -production of Zn2+, disruption of membrane and interference with intracellular components |
S. aureus, E. coli, listeria monocytogenes, Salmonela | Zn NPs |
| -Generation of reactive oxygen species (ROS) -Creating a hole in the bacterial cell wall -DNA binding, DNA degradation and prevent transcription steps -Osmotic balance and integrity of the bacterial cell wall -Penetration into the biofilm -Combined with antibiotics |
Methicillin-resistant S. aureus (MRSA), Proteus mirabilis, A. baumannii ,E. coli, P. aeruginosa, S. aureus | Au NPs |
| -Generation of oxidative stress through the production of reactive oxygen species (ROS) -superoxide radical -hydroxyl radical -hydrogen peroxide -oxygen | S. aureus, E. coli, S. epidermidis | Fe NPs |
| -Generation of reactive oxygen species (ROS) -Cell wall disruption |
E. coli | Al NPs |
| -Changing in Krebs cycle -Interference with the metabolism of amino acids and nucleotides |
Helicobacter pylori | Bi NPs |
| -Prevent energy metabolism -Interference with electron transport chain -Cell wall disruption |
E.coli, Salmonella enteric, E. faecium, Streptococcus spp., Shewanella oneidensis, Acinetobacter baumannii, Burkholderia cepacia, Yersinia pestis, and K. pneumonia | carbon-based NPs |
| -Generation of reactive oxygen species (ROS) -Interference with biochemical processes within bacterial cells |
S. aureus, E. coli, B. subtilis | CuO NPs |
| -Generation of reactive oxygen species (ROS) - Prevent the growth of bacteria |
E. coli, P.aeruginosa, S. aureus | Se NPs |
Factors affecting the Antibacterial Mechanisms of Nanoparticles
Physicochemical properties of nanoparticles including size, charge, zeta potential, surface morphology, and crystal structure, are important factors regulating the performance of nanoparticles on bacterial cells. Also, environmental conditions and duration of exposure to other factors affect the antibacterial effects of nanoparticles. (65). Current research has shown that the size of a nanoparticle greatly affects its antibacterial activity. Smaller nanoparticles have more specific surface areas and are more likely to come in contact with bacterial cell membranes compared to large nanoparticles and polymers (66). Since the shape is an important factor in antimicrobial activity, nanoparticles with different shapes can damage bacterial cells by interacting with pre-plasmic enzymes. Although nanoparticles are reported to have different forms such as bars, wires, tubes, scales, and discs, they are mostly seen spherically. Nanotubes and nanorods seem to be more effective than other forms to kill the bacteria. This indicates that the effect of antibacterial activity is due to the specific surface area (67-68). By increasing the roughness of nanoparticles, the size and ratio of surface to mass causes the absorption of bacterial proteins and consequently reduces the bacterial adhesion of nanoparticles (69). Recent studies have shown that surface charge in nanoparticles has a significant impact on the association between bacteria and nanoparticles. This connection is due to the electrostatic attraction between positively charged nanoparticles and negatively charged bacterial cell membranes. However, at higher concentrations, negatively charged nanoparticles have a good level of antibacterial activity due to molecular overcrowding, which leads to proper interaction between nanoparticles and bacterial cells (70). For example, positively charged polystyrene nanoparticles disrupt the electron transfer chain in bacteria. A study on E. coli bacteria also reported that bacteria with mutations in the ubiquinone gene were more sensitive to positively charged nanoparticles (67). A wide range of studies has also shown that different environmental conditions cause significant differences in the antimicrobial activity of nanoparticles. For instance, ambient temperature has different impacts on antibacterial activity due to its effects on the production of reactive oxygen species. Additionally, the pH of the environment affects antimicrobial activity. It appears that acidic conditions bind nanoparticles to the bacterial cell walls. Furthermore, for example with decreasing pH, the rate of dissolution of zinc oxide nanoparticles increases, which causes an increase in antimicrobial properties (73). According to studies, nanoparticles are a good alternative to antibiotics and have a high potential to solve the problem of the emergence of resistant bacteria; because some nanoparticles have no cellular toxicity or they have very low toxicity. Also, new methods for nanoparticle production do not involve high-risk and complex processes (74).
Research Limits
The antibacterial mechanisms of nanoparticles are still unknown. Many studies, for example, attribute antibacterial activity to oxidative stress or reactive oxygen species, while for some nanoparticles, including magnesium oxide nanoparticles, the antibacterial mechanism may not regulate bacterial metabolism; therefore, one of the issues to be addressed in future studies is to further investigate the antibacterial mechanisms of nanoparticles. Lack of integrated standards is another limitation regarding antibacterial mechanisms of nanoparticles. In particular, the use of different bacterial strains, operating time, and nanoparticle properties have not been standardized in various studies, which makes it difficult to compare antibacterial activities. In addition, sensitive bacterial strains are often used to accurately determine the antibacterial activity of nanoparticles. Other limitations include the complex structure of bacterial cell membranes and the lack of research methods for their laboratory studies. Moreover, laboratory models cannot replicate in vivo conditions accurately; therefore, it is impossible to estimate the antibacterial performance of nanoparticles through laboratory bacterial cell culture alone. Many questions remain unanswered, questions regarding the toxicity of nanoparticles as well as how nanoparticles pass through the bacterial cell membrane which all will need to be answered in future researches.
Since in recent years antibiotic resistance has accelerated, fighting infectious diseases and treating patients have become challenging, which leads to serious complications and death. According to several studies, nanoparticles are a suitable replacement for antibiotics and it seems they could solve the problem of the emergence of resistant bacteria; since nanoparticles either have no cytotoxicity or are usually very low in toxicity. Also, their production methods do not involve high-risk and complex processes. Nowadays, with the help of green chemistry, smaller and more efficient nanoparticles are produced by simple and clean methods with acceptable antibacterial properties (71).
We hope that this review study can provide a better perspective for researchers and an in-depth study of the antibacterial mechanisms of nanoparticles will lead to the production of effective antibacterial particles with no cytotoxicity.
The technical support for this work has been provided by Mashhad University of Medical Sciences.
The authors declare that they have no conflict of interest.
Authors declared no conflict of interests.
Received: 2020/05/3 | Accepted: 2020/11/10 | ePublished: 2021/01/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |