BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1121-en.html

 , Minoo Aliabadi2
, Minoo Aliabadi2 
 , Sepideh Kadivarian2
, Sepideh Kadivarian2 
 , Soroush Borji2
, Soroush Borji2 
 , Jale Moradi3
, Jale Moradi3 
 , Amirhooshang Alvandi4
, Amirhooshang Alvandi4 


2- Student Research Committee, and Department of Microbiology, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
3- Medical Technology Research Center, Research institute for Health Technology, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
4- Medical Technology Research Center, Research institute for Health Technology, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran ,
Introduction
According to the definition by the world health organization (WHO) and food and agriculture organization (FAO), probiotics are live non-pathogenic microorganisms that have beneficial effects on the health of human if consumed sufficiently (1). A main group of these microorganisms is lactic acid bacteria (LAB) that are used as starter for dairy products. Probiotic strains of LAB have antioxidant, anticancer and antimicrobial potential effects. Moreover, it has been proved that probiotic bacteria can reduce serum cholesterol, relieve lactose intolerance, and stimulate the immune system leading to the stability of the microbiota (2). LAB that are associated with ferm-ented foods include some species of Carnobacterium, Enterococcus Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Tetragen-ococcus, Vagococcus, and Weissella genera (3).
Fermentation is a frequently used food processing technique worldwide. There are many locally produced fermented foods (4) (5), including various kinds of yogurt, cheese, fermented milk, butter milk, curd, and butter (5). Some evidences suggest traditional butter fat or Ghee is a fermented food and could contain different kind of LAB. Fermentation of milk to yoghurt is traditionally occurred by Lactob-acillus bulgaricus and Streptococcus thermophiles at 40-45°C. These bacteria, are not present in intestinal microbiota, are not s and cannot survive in the gut (6).
Changes that occur during milk fermentation by LAB enhance nutritional value of the product (7). Kermanshahi traditional oil, which is traditionally produced, serves as an imp ortant type of fat in the diet of some areas in Iran and other countries of the South Asia (8). It is known as yellow oil, Kermanshahi oil, Ghee, roghan-e heiwâni, or Kermanshahi traditional oil (9). First, yogurt is produced from milk by traditional methods, and then it is put in a Mashk (a large leather bag/a big waterskin). After this step, butter is separated from the tan (doogh), then transferred to a larger pot, and slowly heated, allo-wing the components to separate by density and the remaining is called roghan-e heiwâni. Studies have shown that changes traditionally occur in the preparation of butter and Kermanshahi traditional oil in fatty acid. Among the reasons of such changes, bacterial fermentation process for the preparation of products can be pointed out. In products like yoghurt, butter and Kermanshahi traditional oil, due to the function of bacteria, lactic acid is produced and envir-onmental pH is reduced (10). Furthermore, changes occur in the fatty acid of these products. There has been no study on isolating probiotics from Kerman-shahi traditional oil preparation stages. Therefore, our purpose in this study was to identify probiotic bacteria from these products using culture and PCR-sequen-cing. Recognizing and recording of these microorga-nisms can help to preserve biological sources.
Materials and Methods
The present study was conducted during the period from October to the end of November, 2015. A total of 15 samples from each dairy product including yogurt, butter and Kermanshahi traditional oil were collected from Kermanshah Province. The samples were collected in sterile bottles and kept cold until they arrived at the laboratory, where they were kept at 4°C. Only samples have been prepared using traditional starter were included in the study to ensure the absence of commercial starters. Ten grams of each sample were homogenized with 90 mL sterile normal saline solution (0.85%, w/v) to make an initial dilution (10-1). An amount of 100 µL from each diluted sample was then cultured on MRS Agar (Oxoid, CM0361) and M17 Agar (Oxoid, CM0785) containing 0.05% cysteine, and incubated in both aerobic and anaerobic conditions at 37°C for 48-72 hours. All bacterial isolates were screened using colony morphology and gram staining, and final identification was carried out through PCR sequencing. Then, 4-5 colonies were selected from each plate. After subculture, DNA of each plate was isolated using the DNA extraction kit according to the manufacturers’ instruction. The extracted DNA was stored at -20 until PCR implementation. The PCR technique was employed for the amplification of genus-specific target of Lactobacillus and Bifidobacterium of using specific primers and universal primer of 16S rRNA gene previously described by Lauren et al. The primers used in this study are summarized in Table 1 (11). The PCR was performed in a total volume of 25 μL, containing 2.5 μM dNTP mix, 2.0 mM MgCl2, 0.5 U Taq DNA polymerase, 0.5 μM of each primer, and 2.5 μL of each template DNA and 10X buffer. Standard strains, Lactobacillus delbrueckii (PTCC 1737) and Bifidobacterium bifidum, (PTCC 1644) were used as a positive control in each reaction. The PCR was performed under the following conditions: Denat-uration for 5 min at 95°C; 30 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C, as well as a final extension step of 7 min at 72°C. The PCR products were analyzed by electrophoresis on a 1.5% agarose gel. The agarose gel was stained using ethidium bromide and visualized in gel doc. Visualization of 340 bp and 520 bp confirmed the Lactobacillus and Bifidobacterium genus, respectively. Finally, each sample with single band within 470 bp was selected for 16SrRNA sequencing. The nucleotide sequences of the 16SrRNA `gene of all the isolates were analyzed and edited using Bioedit software. Similarity searching was conducted for each edited sequence for exact determination of genus and species of each isolate (http://www.ncbi.nlm.nih.gov/BLAST). All 16SrRNA sequences were submitted to the NCBI GenBank under the accession numbers KX951698.1 To KX951735.1, KX926497.1 to KX926536.1.
Table 1. primer sequences for PCR-based detection of probiotic bacteria
| Target | Primer Name | Sequence (5ʹ to 3ʹ) |
| Universal primers 16SrRNA gene | 341 –F 768-R |
CCTACGGGAGGCAGCAG GACTACCAGGGTATCTAATC |
| Specific primers Lactobacillus 16SrRNA gene | Lact-F Lact-R |
AGCAGTAGGGAATCTTCCA ATTYCACCGCTACACATG |
| Specific primers Bifidobacterium 16SrRNA gene | Bif-F Bif-R |
GGGTGGTAATGCCGGATG CCACCGTTACACCGG |
Results
Overall, 45 samples were cultured and 78 colonies were selected according to colony morphology. All the isolates were gram positive catalase and oxidase negative. The isolates were all confirmed using the PCR and sequencing targeted 16SrRNA gene. The result is shown in Figure 1 and Table 2. Several species were identified including Lactobacillus, Lactococcus, Enterococcus, Streptococcus and Bacillus from the samples. The result showed that Lactobacillus was the dominant flora of the existing probiotic microorganism in the samples. In the yogurt samples, the L. delbrueckii, Lactobacillus helveticus, Lactob-acillus fermentum, Lactobacillus rhamnosus, Lactob-acillus paracasei with Streptococcus thermophilus, Enterococcus faecium, Brevibacillus formosus and Brevibacillus agri species were isolated. Brevibacillus and E. faecium were isolated from the yogurt samples only. Also, Brevibacillus was isolated from two samples only. However, L. paracasei and L. rhamnosus were isolated from both the yogurt and butter samples. Several species of Lactobacillus, Enteroco-ccus, Streptococcus, Lactococcus and Bacillus were also isolated from the butter samples. The most prevalent genus was L. delbrueckii, L. helveticus, L. paracasei, L. rhamnosus, and L. fermentum. Other bacteria that were isolated from the traditional butter samples with less frequency were Lactococcus lactis, S. thermophiles, Enterococcus feacalis and Bacillus cereus. Bacillus subtilis and Bacillus licheniformis were isolated only from the butter samples. The detailed results of the isolates are shown in the following Table 2. Some species of the Lactobacillus, Streptococcus and Enterococcus genus were present in Kermanshahi traditional oil, but in a small number. The Lactobacillus species included L. fermentum, L. delbrueckii, Lactobacillus casei and L. helveticus. S. thermophiles, Enterococcus feacalis, Enterococcus feacium and Enterococcus hirae were the other isolated species from Kermanshahi traditional oil. E. hirae, however, was isolated from a single Ghee sample. The frequency of probiotic bacteria in all the samples including local yogurt, butter and Kermanshahi traditional oil was %84.44 for Lactobacillus, %57.78 for Streptococcus, %11.11 for Enterococcus and %15.56 for Bacillus.
Table 2. probiotic species in collected samples
| Bacteria | Bacteria isolated Kermanshahi traditional yogurt(n)% | Bacteria isolated Kermanshahi traditional butter(n)% | Bacteria isolated Kermanshahi traditional oil (n)% | Total |
|---|---|---|---|---|
| L. delbrueckii | (9) 60 | (6) 40 | (4) 26.67 | (19) 42.22 |
| L. helveticus | (1) 6.67 | (4) 26.67 | (2) 13.33 | (7) 15.56 |
| L. fermentum | (2) 13.33 | (2) 13.33 | (2) 13.33 | (6) 13.33 |
| L. rhamnosus | (1) 6.67 | (1) 6.67 | 0 | (2) 4.44 |
| L. paracasei | (1) 6.67 | (2) 13.33 | 0 | (3) 6.67 |
| L. casei | 0 | 0 | (1) 6.67 | (1) 2.22 |
| S. thermophilus | (11) 73.33 | (4) 26.67 | (11) 73.33 | (26) 57.78 |
| Bervibacillus | (2) 13.33 | 0 | 0 | (2) 4.44 |
| E. faecium | (1) 6.67 | 0 | 0 | (1) 2.22 |
| E. faecalis | 0 | (1) 6.67 | (2) 13.33 | (3) 6.67 |
| B. cereus | 0 | (1) 6.67 | 0 | (1) 2.22 |
| L. lactis | 0 | (2) 13.33 | 0 | (2) 4.44 |
| E. hirae | 0 | 0 | (1) 6.67 | (1) 6.67 |
| B. subtilis | 0 | (1) 6.67 | 0 | (1) 6.67 |
| B. licheniformis | 0 | (3) 20 | 0 | (3) 20 |
| Total | (28) 100 | (27) 100 | (23) 100 | (78) 100 |
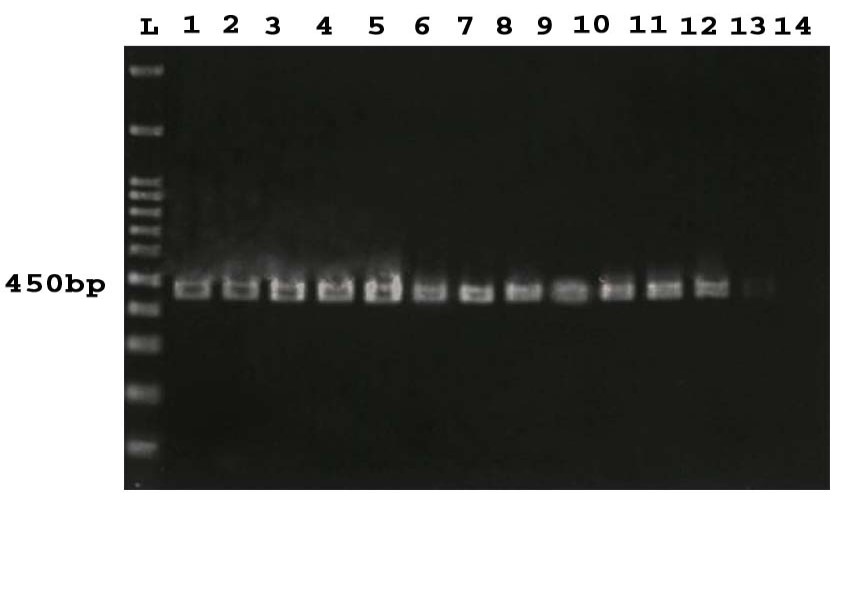
Figure 1. PCR products of 16SrRNA gene. L: 100 bp DNA Marker, 1-13: positive samples, 14: Negative control.
Discussion
Probiotic bacteria are non-pathogenic microorga-nisms known for their nutritional value and thera-peutic effect as well as anti-microbial and anti-cancer effects on the host health. During the past two decades, these microorganisms have been isolated from variety of foods, especially dairy products. A wide range of dairy products such as fresh milk, yogurt, cheese, fermented butter and drinks containing LAB can provide a good environment to support the growth and durability of probiotic microorganisms (12, 13). Many studies have been conducted on the isolation and identification of probiotic bacteria in various traditional dairy and fermented food products from all around the world (14). Lactobacillus plantarum, L. casei and Lactob-acillus brevis were isolated from (traditional) yogurt and cheese by Ebrahimi et al. (15). Angmo et al. succeeded in isolating L. plantarum from Ladakh, a fermented vegetable product in India (1). Seven species of Lactobacillus and Bifidobacterium were isolated and reported studying traditional fermented dairy products in different regions of Russia (16). Moreover, numerous studies have indicated that isolated lactic acid bacteria from milk, cheese, fermented olives, kefir, sourdough of wheat and barley flour and other fermented products have the ability to inhibit the progress of some gastrointestinal diseases and have remarkable antibacterial properties compared with commercial strains (17-23). The most important lactic acid bacteria in the dairy industry are Lactobacillus, Streptococcus, Lactococcus and Entero-coccus, and according to many studies, traditional fermented products are mostly dominated by Lactob-acillus species (24). In the present study, Lactobacillus was the dominant 84.44% of all lactic acid bacteria. L. delbruekii and S. thermophilus were reported to be the most abundant probiotic bacteria in a Colombian study of dairy products using biochemical tests and 16SrRNA gene sequencing (25). Lactobacillus acidophilus and L. casei were reported as the most abundant isolate from dairy products of Bhardwaj in India (26, 27). L. paracasei and L. rhamnosus, two species that were isolated from yogurt and butter in present study, were used commonly as non-starter lactic acid bacteriain in dairy products. In some studies, L. paracasei has been isolated and identified from cheese and curd samples. Bervibacillus brevis and E. faecium were isolated from yogurt samples in this study. Although similar results have been reported (28), little information is available about their probiotic properties, which might even be regarded as contamination. Several reports have been presented in isolating (25) probiotic bacteria from local yogurt and butter separately. However, this study was the first to focus on isolation and identification of LAB from traditional Kermanshahi oil in various stages of development. Kermanshahi traditional oil is a common edible oil in parts of Iran including Kermanshah Province. It is an important consumable fat in people’s diet in particular areas in Iran, and is traditionally prepared from yogurt (29). Bahrami et al. showed that fatty acids can change in traditional preparation of butter and Kermanshahi traditional oil. One of the reasons can be the fermentation process and lactic acid production by probiotic bacteria (10). Lactobacillus, Enterococcus, Lactococcus and Leuconostoc genera were isolated from traditional butter (Dhan), in a previous study (30). Thus, a probiotic bacterium in the preparation process of fermented products has been isolated according to traditional methods without the use of any commercial starter, which can be a valuable sou-rce of probiotic microorganisms. Hence, preparation of fermented products is being industrialized, making isolation and preservation of local strains particularly valuable. In this study, we attempted to isolate bacteria from local traditional yogurt, butter and Kermanshahi traditional oil. The result of this study revealed different isolated groups of probiotic bact-eria from the samples of traditional yogurt, butter and Kermanshahi traditional oil, indicating that fermentation conditions were different in the samples. We found that L. delbruekii, L. helveticus, L. fermentum, and S. thermophilus were the pred-omin-ant isolates from the samples of traditional yogurt, butter and Kermanshahi traditional oil. Tarhana is a traditional fermented food mixture of wheat flour and yoghurt. During natural fermentation in Tarhana, LAB are dominant strains while S. thermophiles, L. delbr-ueckii and L. fermentum are dominant isolates (31). In our study, S. thermophilus and L. delbrueckii were the predominant isolates from the three samples. Ferm-entation of lactose by LAB produce large amounts of lactic acid. Consuming ferm-ented Ghee with low pH increases absorption of a short chain of fatty acids essential for the optimum performance of the body (10). L. delbrueckii as a starter with the S. thermophiles species used in dairy industries has shown that all strains have high lactose activities that help to improve lactose digestion and eliminate symptoms of lactose intolerance (32). Lactobacillus delbrueckii and S. thermophiles elimin-ate cholesterols by binding to released bile acids, which can reduce the risk of cardiovascular diseases (33). L. helveticus can hydro-lyze milk proteins and release bioactive peptides that are responsible for elevating IgA in the bronchial and intestinal regions and subsequently, for boosting muc-osal immunity. As a thermophilic bacterium, L. ferm-entum is a non-starter lactic acid bacterium, which has considerable importance in fermented foods. It is isolated from different environments such as fermented milk. L. fermentum is one of the dominant Lactobacillus species in the intestinal tract and vagina in humans (34). In contrast to other studies, we isolated the Brevibacillus species only from yogurt (13.33% from yogurt), where Brevibacillus was also isolated from soil and milk. So far, this species has not been isolated from any food substance in Iran. However, at least in one study, Brevibacillus was used successfully as a starter for curd. It has been confirmed that Brevibacillus can be used as a suitable candidate in fermented milk production (28). The frequency of isolation was 6.67% for L. paracasei, 2.22% for L. casei and 4.44% for L. rhamnosus. These bacteria are frequently used as non-starter LAB in food and pharmaceutical industries. In addition to different species of Lactobacillus, S. thermophilus, L. lactis and B. cereus from traditional butter samples, E. faecium was isolated from two samples of traditional butter and oil in the present study. Bacillus strains are highly resistant to physical and chemical conditions due to spore formation, and are widely used as probiotics. L. lactis isolated from traditional butter in the present study has been indicated as a probiotic bacterium, and its antibacterial effect has been proven against pathogenic bacteria by Garsa earlier in a research (35). The presence of Enterococcus hirae and Enterococcus faecalis in traditional oil samples was another finding of the present study. While about 50% of the lactic acid isolated from Brazilian cheese belonged to Enterococcus species, four strains of E. hirae were isolated from fermented cheese by Xinjisng in another study (27, 36). The genus Enterococcus has many species, but E. faecalis, E. faecium, and E. lactis, and more recently E. hirae and E. dorans have been studied as probiotic species. A strain of Enterococcus, named E. faecium K77D, is used commercially as a starter (37-40). L. rhamnosus has been extensively studied. This bacterium can survive even in difficult conditions of the digestive system and urinary tract (41). Non-starter LAB are traditionally used to improve organoleptic properties of products. Non-starter bacteria such as some genus of Lactobacillus and Enterococcus are resistance to heat and extreme conditions. Guessa et al. predominantly isolated Lactobacillus, Enterococcus, Lactococcus and Lecconestoc species from a traditional butter called "Dhan" (42). Enterococci are not used as starter in food preparation, but they can be used as dietary supplements in pharmaceutical preparation. Never-theless, the use of Enterococci as probiotics has rema-ined unknown in food safety. It must be noted that Enterococci have a positive role in traditionally ferm-ented foods (43, 44).
Conclusion
Various species of Lactobacillus and other lactic acid bacteria were isolated and identified from traditional diary products using culture and 16SrRNA gene sequencing, in this study. L. delbruekii, L. helveticus and L. fermentum are a group of thermophilic bacteria used as starters at high temperatures. Due to their ability to tolerate higher temperatures, in our study, these species were isolated from all three kinds of samples including Kermanshahi traditional oil that receives intense heat in the production process. These species have more suitable proteolytic properties and more beneficial biological effects than other Lactobacillus species. L. fermentum, was isolated from all three kinds of samples, traditional yogurt, butter and oil and is widely used in the production of fermented food. In this study, a variety of potentially probiotic bacteria were isolated from all three samples, but many are probably killed by high temperatures during the preparation and production of traditional oil. However, findings of the present study showed that some species were able to tolerate high heat and harsh conditions, and were able to survive and replicate in traditional oil. Therefore, isolation and identification of various potentially probiotic bacteria from different traditional dairy products can help preserve these bacteria to be used in fermented and functional food products.
Acknowledgements
The authors of the article express their gratitude to the adjutancy Research and Technology of Kermanshah University of Medical Sciences for financing this research project with the tracking code 93323.
Conflicts of Interest
There is no Conflict of Interest.
Received: 2020/05/2 | Accepted: 2020/12/5 | ePublished: 2021/06/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





