BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1113-en.html
2- Department of Veterinary Pathobiology, Faculty of Veterinary Medicine, Islamic Azad University Rasht Branch, Rasht, Iran
3- Department of Animal Science, Faculty of Agriculture, Islamic Azad University, Rasht Branch, Iran
.
Livestock breeding plays an important role in the economy of villages in Guilan province in Iran and it is known as the only source of income for the rural middle class people in this province. Despite the existing problems, livestock breeding in this province is increasingly high and plays a significant role in the national economy (1). The dairy industry always suffers from various diseases, and among them, parasitic diseases and infections are of special importance since they impede the development of the dairy industry (2).
In the livestock industry, under some circumstances, such as low production for unknown reasons, high cost of treatment and workforce, and various parasitic diseases, the extermination of animals is inevitable (3). Control of various parasitic diseases is of the utmost importance in reducing costs and production-related disorders. Different parasitic infectious agents are known to be the cause of respiratory diseases in the livestock population. However, Dictyocaulus viviparus has been identified as a potentially growing and costly problem (4) and the cause of parasitic pneumonia (5, 6, 7). Lungworm (D. viviparus) is a relatively common parasite in tropical and subtropical regions and is the cause of economic losses to the livestock industry. This parasite causes severe lung diseases in cattle, commonly referred to as parasitic bronchitis, D. viviparus, or husk (8). Infected herds, depending on the degree of pasture contamination, usually show a high incidence of disease and mortality (9). Clinical signs in naturally infected animals include decreased appetite and growth, increased respiration and cough (10).
D. viviparus is known as a parasite that causes high mortality in cattle (11). Healthy animals become infected by eating contaminated forage. Chronic inflammatory changes in the animal’s lungs include loss of ciliated epithelial cells, peribronchiolitis, eosinophilic bronchiolitis and atelectasis (12). In Iran, nematode infections in sheep and goats have been reported frequently (13, 14, 15). However, there is limited information on the natural occurrence of D. viviparus infection and its pathology in native cattle and buffaloes. Therefore, the present study was designed to identify the incidence and frequency of macroscopic lesions and histopathology of infection with D. viviparus in dairy cattle and buffalos slaughtered in Rezvanshahr slaughterhouse in Iran.
In Rezvanshahr city, cattle and buffaloes are mainly kept in small groups (less than 10 heads) in rural areas. Dairy cattle are usually fed in a closed system using straw and concentrate, while younger animals graze on newly harvested farmland, around water canals and roadsides. Manual feeding and grazing of animals often take place throughout the year.
This research was conducted in 2018 and the inclusion criterion was the presence of cows and buffaloes infected with D. viviparus in the slaughterhouse. First, 189 male bulls and 212 male cows in Rezvanshahr slaughterhouse were examined for the infection of D. viviparus parasite in the feces. Bulls (n=140) and cows (n = 95) were divided into 3 groups: less than 1 year old, 1-3 years old, and more than 3 years old. Similarly, all bulls (n=125) and cows (n=85) were classified into 3 age groups.
Stool Test
About 25 g of fresh feces were taken from the rectum of each animal before slaughter. The samples were stored at room temperature in the laboratory. Parasite eggs were identified using the Baermann technique and studied under a microscope (13).
Macroscopic and Histopathological Studies
After slaughter, all animals were studied one by one for the presence of lesions in the lungs. Finally, 5 lungs belonging to 5 buffaloes and 5 lungs belonging to 5 cows with symptoms of severe pneumonia, nodular lesions in the lung and hyperemia were identified and selected. The trachea, bronchi, and bronchioles of the animals were carefully dissected and examined for adult lungworms. Damaged lung tissue was treated with 10% stabilized neutral formalin buffer by conventional dewatering and immersion in paraffin. Then, tissue sections with a thickness of 4-5 microns were prepared and finally stained by hematoxylin and eosin (H&D) method and studied under a light microscope (16). Microscopic lesions were examined and scored using a method previously proposed by Jung et al. (2012) (17).
Data Analysis
The collected data in the present study were analyzed using Chi-square test method with about 95% confidence using SPSS software version 16 (SPSS Inc., Chicago, IL., USA).
In the present study, the overall prevalence and infection of D. viviparus was recorded as 20.2%, while the prevalence of this parasite among buffaloes and cows were 26.32% and 22.64%, respectively. However, the difference between the two animal species was not significant. Overall, the prevalence of D. viviparus was higher among young animals in both species. The results are presented in detail in Table 1.
| Number of cattle | Number of positive | Pct. | ||||
|---|---|---|---|---|---|---|
| Cow | 212 | Under 1 year | 102 | 28 | 27.45 | 22.64% |
| Between 1-2 years | 48 | 8 | 16.66 | |||
| Over 2 years | 62 | 12 | 19.35 | |||
| Buffalo | 189 | Under 1 year | 91 | 23 | 25.27 | 26.32% |
| Between 1-2 years | 42 | 3 | 7.14 | |||
| Over 2 years | 56 | 7 | 12.5 | |||
| Total | 401 | 401 | 81 | 20.2 |
Under macroscopic examination, the lungs of infected cows and buffaloes showed nodular appearance, hyperemia, pleural adhesion, and purulent exudate (Table 2). Adult cylindrical worms were abundant within the upper posterior bronchi and within the terminal branches of the posterior bronchioles. The lungs were stiff and foamy exudates were visible in the bronchi.
Table 2. Periodicity of lung lesions due to Dictyocaulus viviparus in cows and buffaloes
| Lesions | Buffalo | Cow | ||
| Number | Pct. | Number | Pct. | |
| Macroscopic | ||||
| Hyperemia | 3 | 60 | 2 | 66.6 |
| Foam in the trachea | 1 | 20 | 0 | 0 |
| Stiffness of lung | 2 | 40 | 1 | 33.33 |
| Nodular | 4 | 80 | 3 | 100 |
| Pleural adhesion | 0 | 0 | 1 | 33.33 |
| Pleural exudation | 1 | 20 | 1 | 33.33 |
| Parasites in the bronchioles | 2 | 40 | 0 | 0 |
| Histopathological | ||||
| Stages of parasitic growth and development (eggs / larval stage L1, adult) | 4 | 80 | 2 | 33.33 |
| Accumulation of mononuclear cells | 5 | 100 | 3 | 0 |
| hyperemia | 3 | 60 | 2 | 66.66 |
| Peribronchial fibrosis | 4 | 80 | 0 | 0 |
| Alveolitis | 4 | 80 | 0 | 0 |
| Peribronchiolar cuffing | 3 | 60 | 1 | 33.33 |
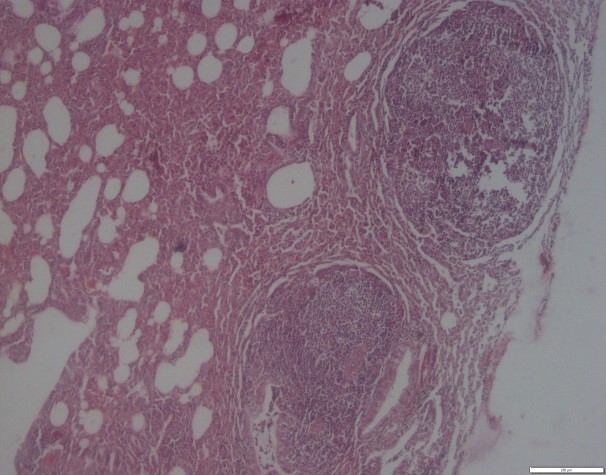
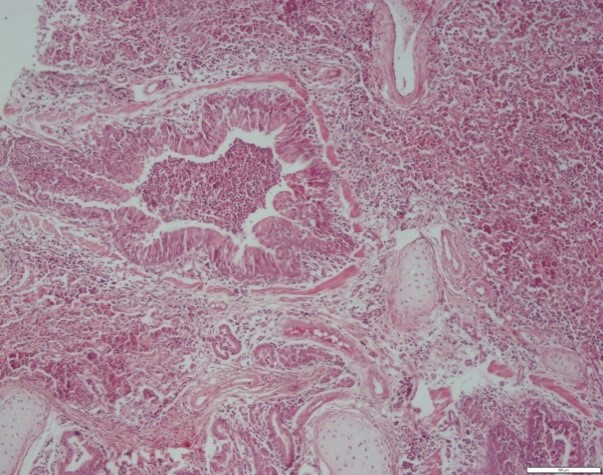
Histopathological studies showed an abundance of exudates within the bronchioles, which mainly contained eosinophils, lymphocytes, macrophages, and giant cells. Degenerative and necrotic changes were observed in the epithelium of bronchioles. Some histopathological sections showed catarrhal bronchiolitis and atelectatic changes along with alveolitis granulomatosis and specific eosinophilic. Hyperplasia of goblet cells and lymphoid tissue around the bronchioles were also observed (Figures 1, 2, 3, and 4).
Figure 1. Cow lung. Bronchioles. Hyperplasia of goblet cells and epithelial cells with exudate containing inflammatory cells. H&E coloring. Magnification 4.
Figure 2. Buffalo lung. Granulomatous alveolitis and atelectatic changes. H&E coloring. Magnification 4.
Figure 3. Buffalo lung. Bronchioles. Hyperplasia of goblet cells and lymphoid tissue around the bronchioles. H&E coloring. Magnification 4.
Figure 4. Cow lung. Bronchus. Exudates of inflammatory and neutrophil-rich cells within the bronchial canal and alveoli. H&E coloring. Magnification 4
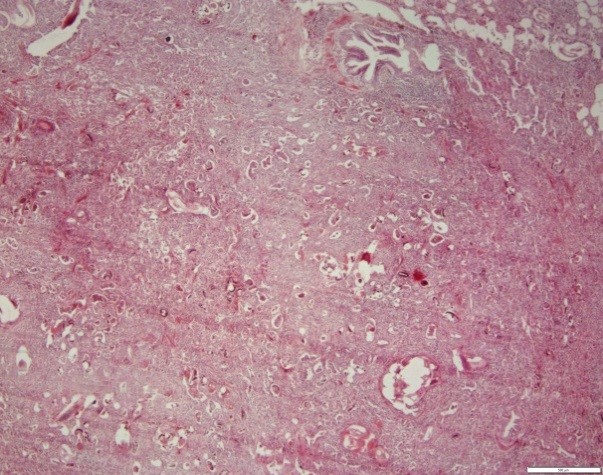
Egg clusters, freshly hatched larvae were observed in the alveoli, and rupture of the inter-alveolar wall in the lungs of cattle and buffaloes. The inter-alveolar walls were thickened due to inflammatory cell infiltration, mild fibroplasia, and proliferation of type II pneumonocytes (Figures 5 and 6).
Figure 5. Cow lung. Note the presence of larval and egg sections of the parasite in the respiratory tract, cellular exudate within the ducts and alveoli, and thickening of the wall between the alveoli. H&E coloring. Magnification 4.
Figure 6. Buffalo lung. Note the presence of larval and egg sections of the parasite in the respiratory tract, cellular exudate within the ducts and alveoli, and thickening of the wall between the alveoli. H&E coloring. Magnification 4.
Previously, the prevalence and infection (34-8%) with D. viviparus parasite in various dairy herds and sheep in different climatic regions of different countries had been reported (18, 19, 20). Heavy rains during hot and humid seasons are suitable for the survival of infectious nematode larvae in green forage pastures that support the growth and development of these nematodes and the greater chance of being eaten by cattle and buffaloes.
Although infection with D. viviparus has been reported from tropical and sub-tropical countries such as Brazil (21), India (22), Malaysia (23), and Turkey (10), countries with a temperate climate, such as Ireland (24), Germany (25), the Netherlands (4), and Sweden (26) are not free of this parasite. Lungworm infection has also been reported among wildlife animals such as roe deer and cervids (27, 28).
In the present study, the prevalence of D. viviparus infection in young animals was insignificantly higher (P>0.210) (Table 1). The reason can be explained by the fact that older animals have grown immunity to the disease and do not repel nematode larvae (29, 30).
Different developmental stages of the parasite, including newly hatched larvae of D. viviparus, have also been reported in ruminants (31). In the present study, chronic inflammatory cells and increased connective tissue proliferation were observed in infected lungs. Loss of bronchial ciliated epithelial cells was observed along with inflammatory cells infiltration and formation of lymphoid follicles around the bronchioles. These pulmonary changes may be due to the proliferation of immunological cells in response to the proliferation of D. viviparus eggs and the migration of adult larvae into lung tissue (10).
Ploeger et al. (2002) reported that D. viviparus, the main etiologic cause of parasitic bronchitis in animals, is first swallowed as a larva and then penetrates the intestinal wall and then passes through the lymph nodes and migrates through the bloodstream to the lungs and becomes an adult worm (4). In the lungs, pathological changes occur due to the invasion and activation of eosinophils and mast cells, which lead to narrowing of the airways and cause edema, emphysema, and alveolar collapse (4). Similar changes have been reported in the lungs due to D. viviparus infection in cattle (25), sheep and goats (12), foal (32), deer calves (32), and Rocky Mountain elk (32).
There was no comprehensive study to determine the prevalence of D. viviparus infection in Iran’s climate. The findings of the present study include useful information about the prevalence of D. viviparus nematode infection and its pathological findings in cattle and buffaloes. Therefore, more epidemiological and molecular studies are needed to identify the characteristics of this parasite in Iran.
The authors of this study would like to thank the experts of Rezvanshahr slaughterhouse and the head of the Microbiology Laboratory of the Islamic Azad University, Rasht Branch, who helped us in performing this research.
Authors declared no conflict of interests.
Received: 2020/04/25 | Accepted: 2020/08/26 | ePublished: 2020/10/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |