BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-989-en.html
2- Food, Drug, Natural Products Health Research Centre, Golestan University of Medical Sciences, Gorgan, Iran ,
The spoilage of bakery products is mainly caused by the growth of molds. Aspergillus, which grows in various bakery products, produces aflatoxin leading to serious health problems in people and irreversible economic damage. Food contamination with aflatoxin is a common problem in the tropical and subtropical regions of the world, especially in developing countries (1). Aflatoxin B1 is considered to be the most toxic aflatoxin and the most potent liver carcinogen produced by some species of the genus Aspergillus. The successful control of mold growth can be regarded as one of the most critical steps to prevent cancer (2).
During the last two decades, researchers have paid great attention to the use of microorganisms with antagonistic and harmless effects. These microorganisms are known as the major biological methods for food preservation that reduce Aspergillus growth and aflatoxins (3). Lactic acid bacteria (LAB) can produce some antimicrobial compounds, including organic acids, diacetyl, acetone, hydrogen peroxide, antifungal peptides, and bacteriocins, which are effective against a wide range of pathogenic and spoilage-causing fungi (4).
Oats (Avena sativa L.), commonly known as Oates, belong to the Gramineae family, which have barley-like fruits with two sharp-edged points. Oat species are kind of weeds that are cultivated in different parts of the world, even in Iran due to their compatibility with different climates (5, 6). The sourdough obtained from this grain can be considered as one of the remarkable sources of LAB.
There are numerous studies on the isolation and identification of LAB from yeast, as well as on the antifungal activity of these isolates. For example, Demirbas et al. (2017) isolated 15 LAB from a traditional sourdough sample and examined their antifungal properties. They showed that Lactobacillus paraplantarum and Lactobacillus paralimentarius had the highest antifungal potential against Penicillium chrysogenum and Aspergillus niger (7). According to a study conducted by Sadeghi et al. (2016), Pediococcus pentosaceus isolated from the sourdough of barley flour and the supernatant obtained by cultivating this lactic isolate against Aspergillus had antifungal properties (8).
The possibility of finding isolates with unique capabilities has always augmented the application of the new methods of identifying and determining the functional properties of LAB in the less studied ecosystems. Fermented food products, such as fermented bran are ideal habitats for LAB. Consequently, the present study aimed to isolate and identify dominant lactic isolates in the sourdough of oat bran and investigate their antifungal influence on A. niger.
This experimental study was conducted in 2019 in the Microbiology Laboratory of the Deputy of Food and Drug Administration in Golestan University of Medical Sciences, Gorgan, Iran. First, barley bran flour was purchased from the suppliers of Gorgan city. Following the determination of some of its characteristics, namely moisture content, protein, and ash based on the previously documented procedures (9), sourdough was prepared.
Sourdough Preparation
In order to prepare sourdough from oat bran flour, based on the efficiency of dough [(dough/ flour)×100], respectively, 450 and 160. Supplying of the proper ratio was followed by fermenting the prepared sourdough at 37°C for 24 h. Next, 20% of sourdough was added to the water and flour mixture every single day (i.e., inoculation) and was left in the oven at 37°C for 24 h (10).
Isolation and Primary Identification of Lactic Acid Bacteria
First, 10 g of each sourdough sample was transferred to 90 mL of Ringer's solution under sterile conditions. Afterwards, a serial dilution of the solution was made in eight tubes containing 9 mL of sterile Ringer's solution until reaching the dilution of 109. Two replications of pour culture were completed on deMan, Rogosa, and Sharpe (MRS) agar from the obtained dilutions and the plates were incubated in a CO2 incubator at 37°C for 48 h in the presence of 10% CO2.
In the next step, several colonies were selected from each plate based on the differences in the morphological characteristics of colonies (e.g., color, shape, being convex or concave, and being superficial or deep) and linear culture was performed for purification and propagation on MRS agar medium. Isolation was followed by the initial detection of lactic isolates using biochemical methods, such as catalase test and gram staining (10).
Identification of Lactic Acid Bacteria by Polymerase Chain Reaction
In addition to the initial isolation and identification of lactic isolates using biochemical and morphological methods, including catalase test and gram staining, LAB identification using polymerase chain reaction (PCR) was conducted with specific primers (11). At this stage, DNA extraction was performed by GeneAll Commercial Kit (South Korea) according to the manufacturer's instructions. The applied primer and PCR reaction conditions were selected according to the method of Young et al. (2012).
The initial evaluation of PCR products by electrophoresis was completed on 1.5% agarose gel applying a voltage of 90 V for 40 min. Following electrophoresis and initial approval, PCR products were sent to Bioneer Corporation, South Korea for sequencing. Afterwards, the obtained sequences were compared with the sequences in the National Center for Biotechnology Information (NCBI) using Blast software and the lactic isolates were identified in terms of genius and species (11).
Antifungal Activity of Lactic Isolates
In order to investigate the antifungal activity of lactic isolates, two lines of 3 cm were cultured at a distance of 2 cm from the activated culture on MRS agar medium. Following incubation for 72 h at 37ºC in 10% CO2, 5 mL of yeast extract glucose chloramphenicol (YGC) medium containing mold spores (104 spore/mL) was added to the plate surface, and it was incubated at 37ºC under aerobic conditions. The diameter of the mold colony was measured daily utilizing ImageJ software until the control plate (i.e., MRS agar and a layer of YGC medium containing mold spores) was completely covered with mold. The lactic isolates that inhibited mold growth were selected (12).
Preparation of Cell-free Supernatants
The lactic isolate suspension with OD600=1 was centrifugation at 10000 rpm and 4ºC for 5 min and the cell-free supernatant was isolated. Afterwards, the antimicrobial effect of organic acids was inhibited and the neutral cell-free supernatant was obtained using 0.1 N NaOH and achieving a pH of 6.5, which was the initial pH of the culture medium (13).
Antifungal Activity of the Supernatants of Lactic Isolates
To determine the antifungal effect of the supernatant, 1%-10% of the cell-free supernatant was added to the sterile YGC medium (45ºC) and it was poured into the plate. The control sample included a YGC medium with 10% sterile distilled water. Following the coagulation of the culture medium, 3 µL of fungal spore containing 104 spore/mL was placed as dots on the surface and at the central part of the plate. The plates were placed under aerobic conditions at 26ºC and the mold growth rate was measured daily until the mold in the control sample completely covered the plate surface. Lactic isolate with the lowest minimum inhibitory concentration (MIC) (i.e., the minimum concentration of supernatant that significantly reduced mold growth, compared to the control sample) on the examined molds was selected (14).
Compounds of the Cell-free Supernatant of Lactic Isolates
The supernatant obtained from the lactic isolate with the highest level of antifungal activity was dried by freeze-dryer. Next, 18 g of the obtained powder was dissolved in 100 ml of sterile distilled water. A total of 20 ml of the resultant solution was added to the ethyl acetate solvent in a ratio of 1:3 and was shaken continuously by hand for 15 min. During this procedure, all organic compounds were transferred to the ethyl acetate phase and this phase was placed on the surface of the aqueous phase.
At this stage, the mentioned phase was slowly isolated from the aqueous phase and this process was repeated twice for better extraction of organic compounds. Ethyl acetate was then evaporated using a rotary evaporator at 65ºC under vacuum conditions and the residual containing organic compounds was isolated (14). To identify the organic compounds, the samples were finally sent to the Chemistry and Chemical Engineering Research Institute of Iran under refrigerated conditions. The compounds of the cell-free supernatant of the lactic isolates were identified using gas chromatography/mass spectrometry (GC/MS) (Agilent, model 7890B, USA). The carrier gas (mobile phase) of the GC/MS was helium with a flow rate of 1 mL/min and the column (fixed phase) was Agilent Hp5Ms with a length of 30 m, an internal diameter of 0.25 mm, and a thickness of 0.25 µm.
Statistical Analysis
The data were statistically analyzed using SPSS software version 16 and Excel version 2007. All tests were performed with three repetitions in a completely randomized design. The means were compared by the LSD test at a 5% level.
Characteristics of Raw Materials and Changes in pH and Titratable Acidity
The results of the chemical tests of oat bran flour, including the percentage of moisture, protein, and ash are given in Table 1.
Table 1. Chemical characteristics of oat bran flour
| Moisture | Ash | Protein |
| 10.24±0.04 | 2.74±0.01 | 13.68±0.01 |
Moreover, the trend of variations in the titratable acidity of sourdough obtained through inoculation during four consecutive days is shown in Table 2. As could be seen, pH decreased with fermentation time and the titratable acidity of the oat bran sourdough augmented. In addition, a comparison of the sourdough titratable acidity values during 72 consecutive hours of inoculation process revealed a significant difference (P<0.05) after 48 h, while there was no significant difference between 48 h and 72 h (P<0.05).
Table 2. pH and titratable acidity changes during the fermentation of oat bran sourdough
| Time | pH | Titrable acidity (ºD) |
| 0 | 6.42±0.05a | 16.45±0.35a |
| 24 | 6.02±0.02a | 20.96±1.15b |
| 48 | 4.92±0.05b | 31.45±0.4c |
| 72 | 4.58±0.06c | 33.35±1.25c |
Isolation and Identification of Lactic Isolates
In order to identify the predominant lactic isolates after 3 days of repeating the sourdough inoculation process, the LAB of each sourdough were isolated as described. The findings of the initial tests confirmed that the isolates were gram-positive and catalase-negative. Furthermore, according to Figure 1, the gel electrophoresis of PCR products confirmed the amplification of a target sequence of 1500 bp. The comparison of sequenced products with the sequences in World Gene Bank helped to identify the isolates L. brevis, L. sakei NBRC 15893, and Enterococcus hirae ATCC9790 with 99%, 95%, and 97% similarity, respectively. Moreover, P. pentosaceus ATCC 20336 isolate was found to have a similarity of 92%.
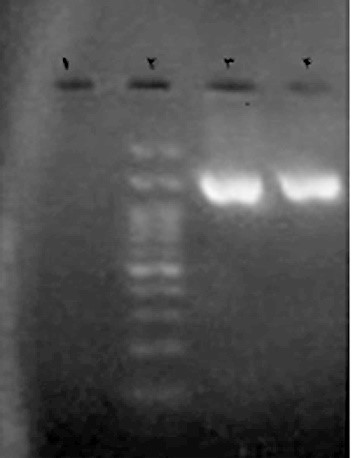
Figure 1. Gel electrophoresis of PCR products and a specific primer with the target sequence of 1500 bp to identify the lactic isolate (line) in the vicinity of the marker of 100 bp (Line 2), positive control samples containing DNA from pure Lactococcus lactis culture (PTCC 1336) (Line 3), and negative or bactericidal control (Line 1)
Effect of Lactic Isolates on the Growth of A. niger
The antifungal activity of lactic isolates against A. niger is demonstrated in Table 3. According to Table 3, A. niger covered the whole surface of the control plate at the end of the fourth day, while the growth of the fungi in the presence of lactic isolates varied from 69.75%-100%.
Table 3. Growth percentage of A. niger in the presence of lactic isolates during 4 days of incubation
| Sample | Day | ||||||
| 1 | 2 | 3 | 4 | ||||
| Control | 10.56±1.42Ad | 56.05±5.86Ac | 95.32±2.11Ab | 100±0.0Aa | |||
| L. brevis | 0.0±0.0Bc | 0.0±0.0Cc | 20.25±4.8Eb | 69.75±3.07Ca | |||
| L. sakei | 0.0±0.0Bd | 7.1±2.41Dc | 37.33±1.59Db | 81.53±4.12Ba | |||
| E. hirae | 0.0±0.0Bd | 51.03±5.02Ac | 86.48±5.76Bb | 100±0.0Aa | |||
| P. pentosaceous | 0.0±0.0Bd | 29.45±2.35Bc | 75.44±3.91Cb | 100±0.0Aa | |||
* Similar lowercase letters in each row indicate that there is no significant difference between the means (P<0.05)
* Similar capital letters in each column indicate that there is no significant difference between the means (P<0.05)
According to the results presented in Table 3, at the end of the fourth day, two of the four studied lactic isolates did not impose a significant impact on the reduction of A. niger growth, compared to the control plate. However, L. brevis and L. sakei had the highest inhibitory effects of 30.25% (Figure 2) and 18.47% against A. niger, respectively. Therefore, the antifungal influence of their supernatants was investigated.
According to the results of Table 4 and Figure 3, the MIC of the raw supernatants of L. brevis and L. sakei against A. niger was 3% with L. brevis having a higher inhibitory potential than L. sakei at all three supernatant levels.
Table 4. Growth percentage of A. niger in the presence of the raw supernatant of lactic isolates
| Raw supernatant (%) | L. brevis | L. sakei |
| Control (0) | 100±0.0Aa | 100±0.0Aa |
| 1 | 100±0.0Aa | 100±0.0Aa |
| 2 | 100±0.0Aa | 100±0.0Aa |
| 3 | 66.33±8.78Bb | 97.65±2.05Aa |
| 4 | 47.93±5.35Bc | 94.18±2.16Ab |
| 5 | 34.14±8.65Bc | 89.72±3.55Ac |
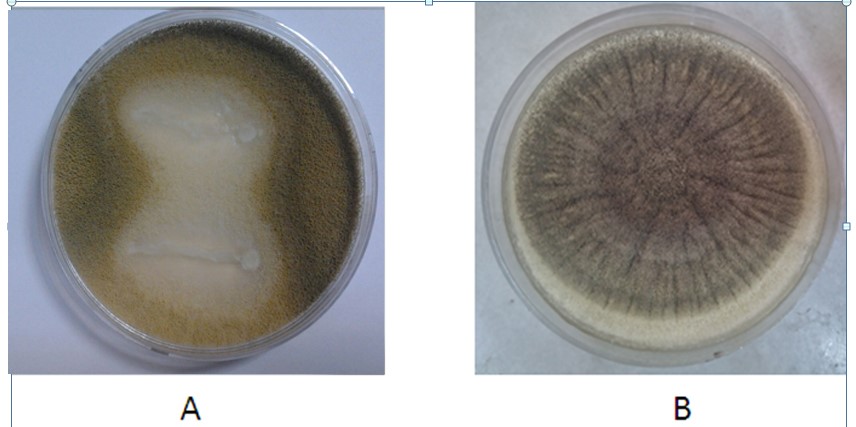
Figure 2. Antifungal effect of L. brevis (A) against A. niger and comparison with the control sample (B)

Figure 3. A. niger growth in the presence of 3% L. brevis supernatant (B), compared to the control sample (A)
Raw Supernatant Organic Compounds
As described, our findings indicated that L. brevis raw supernatant had a more remarkable antifungal effect. Consequently, the compounds of the raw supernatant of this bacterium were studied and identified using GC/MS. The results revealed 14 compounds in raw supernatants, such as fatty acids, esters, phenols, and barbiturates (Table 5).
Table 5. Organic compounds identified in raw supernatant
| Compound | Group | Retention time | |
| 1 | Lactic acid, 3-phenyl-methyl ester | Ester | 13.691 |
| 2 | n-Decanoic acid | Fatty acid | 13.883 |
| 3 | Benzenepropanoic acid, a-hydroxy-methyl ester | Ester | 15.231 |
| 4 | Acetic acid | Fatty acid | 19.754 |
| 5 | 5-Ethyl-5-isopropylpyrimidine-2,4,6(1H,3H,5H)-trione | Barbiturates | 20.202 |
| 6 | Pentadecanoic acid | Fatty acid | 20.458 |
| 7 | Palmitic acid | Fatty acid | 20.914 |
| 8 | 1-Mono-linolein | Fatty acid | 22.373 |
| 9 | 9-Hexadecenoic acid | Fatty acid | 22.652 |
| 10 | Decanedioic acid dibutyl ester | Ester | 24.343 |
| 11 | Hexanedioic acid mono(2-ethylhexyl) ester | Ester | 24.544 |
| 12 | Octadecanoic acid 9,10-dihydroxy-methyl ester | Ester | 24.778 |
| 13 | N-Formyl-D-phenylalanine | Phenol | 25.109 |
| 14 | 5-Ethenyl-5-pentan-2-yl-1,3-diazinane-2,4,6-trione (vinilbital) | Barbiturates | 26.882 |
Developed countries have seriously explored and recognized the novel species of LAB for industrial applications and have used these bacteria extensively for about a century. However, Iran has only addressed this subject during recent years. Therefore, considering many unknown ecosystems, especially microbial, fundamental research should be performed as the first step toward creating an acceptable microbial bank from the LAB in the pristine regions of Iran. Following the research examination, these isolates can be used industrially.
This group of bacteria can produce antimicrobial compounds, such as organic acids, diacetyl, acetone, hydrogen peroxide, antifungal peptides, and bacteriocins, which are effective against a wide range of pathogenic and spoilage-causing fungi (8). In the meantime, oat bran is considered as a source of soluble dietary fiber, which has long been used in breakfast cereals or bakery products. In addition, sourdough is regarded as one of the most important sources of LAB. Nowadays, the microbial flora of fermented products and cultivation-based methods is identified and classified using molecular techniques, including PCR (15).
In the current study, four types of LAB, namely L. Brevis, L. sakei, E. hirae, and P. pentosaceus were isolated from the sourdough of oat bran and were identified by some primary tests, such as gram staining and catalase test followed by the sequencing of PCR products. Similar to the present study, Gobbetti et al. (1998) managed to isolate L. brevis, L. plantarum, and L. fermentum from a sample of wheat flour sourdough as the dominant isolates (16). Holzapfel and Wood (1995) reported L. brevis and L. fermentum as the dominant bacteria in sourdough (17).
Katina et al. (2012) in another investigation encountered a predominantly different microbial population of obligate heterofermentative species, such as Weissella cibaria and W. confuse during the spontaneous fermentation of wheat bran (18). Simsek et al. (2006) evaluated 60 sourdough samples collected from traditional Turkish bakeries. They isolated L. acidophilus, L. plantarum, L. viridescens, L. brevis, L. delbrueckii, L. sakei, and various other species of Lactobacillus and Pediococcus. In a study conducted on the biodiversity of LAB in French sourdough (19), Robert et al. (2009) found that 38% of the isolated LAB belonged to Pediococcus genus (20).
Concerning the antifungal activity of lactic isolates obtained from the sourdough of barley bran against A. niger, we found that L. brevis and L. sakei isolates had higher inhibitory potential. The inhibitory impact of this bacteria on the growth of the studied fungus can be attributed to the influence of produced metabolites on germination and fungal growth, compared to the control sample (21).
Our results are consistent with the findings of Rejiniemon et al. (2015) who showed that lactic acid isolates obtained from malt fermentation products can significantly inhibit the growth of Aspergillus, Penicillium, and Fusarium by producing phenyllactic acid and 4-hydroxy phenyllactic acid (22). Diase et al. (2014) evaluated the antifungal effect of L. brevis as an inhibitor of Aspergillus growth. Their findings suggested that lactic isolates could delay the growth of Aspergillus fungi. They attributed this property to the high production of organic acids by this lactic isolate, such as phenyl lactic acid, 4-hydroxyphenyl lactic acid, and other antifungal protein compounds (23).
Manini et al. (2016) stated that L. brevis isolated from the sourdough of wheat bran had an effective potential to inhibit the growth of A. niger. This inhibitory property on the growth of the studied fungi might be due to the effect of its metabolites on the germination and growth of the fungi (24).
Coda et al. (2011) evaluated the antifungal activity of LAB, including L. plantarum 1A7 and Wickerhamomyces anomalus LCF1695 on the fungi Penicillium roqueforti DPPMAF1 that can elevate the shelf life of wheat bread. Among these isolates, only L. plantarum 1A7 could inhibit Penicillium fungi and fungal contamination was not observed in the bread produced with sourdough containing this lactic isolate for up to 7 days (25).
Yan et al. (2016) conducted research on the antifungal activity of L. plantarum against P. roqueforti under laboratory conditions as a preservative in the processing of Chinese steamed bread. These authors indicated that L. plantarum had an appropriate inhibitory potential on the growth and germination of Penicillium spores. According to the results of the mentioned study, acetic acid and phenyllactic acid produced by this lactic acid isolate were the most important antifungal factors of this isolate. Chinese steamed bread prepared using this lactic isolate had a suitable sensory characteristic for up to 7 days and no fungal contamination was observed in it (26).
Fungal spoilage of bakery products could lead to severe economic loss and health damage to any country. The loss of bakery products, on one hand, and the risks posed by the production of mycotoxins to human health, on the other hand, have prompted researchers to conduct some studies on the use of microorganisms or their metabolites for preventing fungal spoilage and improving food shelf life.
According to the results of the present study, among the four studied lactic isolates, L. brevis and L. sakei had the highest inhibitory effects of 30.25% and 18.47% against A. niger, respectively. Consequently, the antifungal effect of the supernatant of these species was examined. The MIC of a supernatant deterrent for L. brevis and L. sakei against A. niger was 3%.
Considering the greater effect of the supernatant of L. brevis, the compounds of its supernatant were identified using GC/MS. We observed that it consisted of 15 compounds, including ester, phenol, and barbiturates. Therefore, it can be concluded that the predominant lactic isolates of oat bran sourdough have proper antifungal influence against A. niger making it suitable as a starter culture or mobile culture in the production of fermented products, such as sourdough to reduce fungal spoilage.
The authors would like to thank the esteemed experts of the Food Control Laboratory of Golestan University of Medical Sciences for their sincere cooperation in conducting this research, as well as the professors and officials of Saee Higher Education Institute of Gorgan for their assistance.fectious diseases experts for their valuable comments.
Authors declared no conflict of interests.
Received: 2019/11/5 | Accepted: 2020/05/15 | ePublished: 2020/10/5
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







