year 13, Issue 3 (July - August 2019)
Iran J Med Microbiol 2019, 13(3): 220-231 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abdi P, Mahdavi Ourtakand M, Honarmand Jahromy S. The Effect of Matricaria chamomilla Alcoholic Extract on Phenotype Detection of Efflux Pumps of Methicillin Resistant Staphylococcus aureus (MRSA) Isolated from Skin lesions. Iran J Med Microbiol 2019; 13 (3) :220-231
URL: http://ijmm.ir/article-1-950-en.html
URL: http://ijmm.ir/article-1-950-en.html
1- Department of Microbiology, Faculty of Biological Sciences, Varamin- Pishva Branch, Islamic Azad University, Varamin, Iran
2- Islamic Azad University ,masumehmahdavi@gmail.com
2- Islamic Azad University ,
Keywords: Matricaria chamomilla, Phenotype, MRSA, Antimicrobial Drug Resistance, Antimicrobial Agents.
Full-Text [PDF 997 kb]
(2308 Downloads)
| Abstract (HTML) (5577 Views)
Staphylococcus aureus, in particular, methicillin resistant S. aureus (MRSA), is one of the main causes of hospital infections and community-acquired infections, with high morbidity and mortality rates worldwide (1). S. aureus infections cause difficult conditions to care and treat ulcers, damaged skin and soft tissue (2). MRSA are the principle cause of colonization and infection in the acute and chronic ulcerous lesions (4). Efflux Pump is one of the mechanisms of these bacteria in order to show antibiotic resistance (5). Many studies have investigated the inhibitory effect of some plant essential oils on efflux pumps in S. aureus strains (8-10). The simple way to evaluate the phenotypic effect of the efflux pump is the use of EtBr-Agar, which takes advantage agar plates and contains EtBr-increasing concentrations, which is known as the EtBr-Agar or cartwheel method (11, 12). Inside the plants, some compounds act in association with the antimicrobial agents, as they are responsible for disrupting the function of the efflux pumps in plant pathogens (7). Some plants have been involved in the treatment of skin diseases, and their effects have been proven like Matricaria chamomilla belongs to Asteracae family. This plant has antimicrobial and anti-inflammatory effects (13,14). The aim of this study was to investigate the effect of M. chamomilla alcoholic extract on phenotypes of MRSA strains isolated from skin lesions.
This study was performed on 30 samples of S. aureus collected from patients with skin lesions who referred to Shariati hospital in Tehran in the spring of 2017. The samples were characterized by different biochemical tests, including Gram stain, catalase, coagulase, DNase and mannitol fermentation, which led to identification and isolation. The antibiotics resistance of strains was assessed by disk diffusion method. To detect the phenotypic resistance of methicillin-resistant S. aureus, the susceptibility of the bacterial specimen to Cefoxitin disc (30 μg) in the Muller Hinton Culture medium was investigated. The M. chamomilla capitols were collected from Ardebil city and after taxonomic identification, they dried at 25°C in the shade and then powdered by the mechanical mill. The powder obtained from chamomilla capitols was mixed with 80% ethanol and then placed in a Soxhlet extractor. M. chamomilla alcoholic extract was prepared and MIC of it was obtained against MRSA strains by broth microdilution method. In order to investigate the activity of the efflux pumps phenotypically, all stains were evaluated by ethidium bromide containing agar using Cartwheel method (16). After identification of the strains with an activated efflux pump, the effect of M. chamomilla extract on the inhibitory activity of the pump was investigated. For this purpose, the dilutions 1/2 and 1/4 of the MIC of the extract were applied (according to the MIC level of each strain).
According to the results, the highest antibiotic resistance of S. aureus strains was respectively related to penicillin (83.3%), cefoxitin (33.3%), while the lowest resistance belongs to chloramphenicol which reported (3.3 %). among 30 S. aureus strains, 10 strains (33.3%) were MRSA (Fig. 1). The results of this study and the comparison with other reports on the prevalence of methicillin-resistant strains indicate increased methicillin-resistant strains, one of the reasons of which has been due to the excessive use of antibiotics in recent years. MIC of M. chamomilla extract was tested for the studied strains and result obtained between 128-64 μg/ml. The antimicrobial effect of M. chamomilla extract and essential oil against a range of bacteria and fungi has been investigated and confirmed (20, 21).
In this study, ethidium bromide cartwheel assay was performed to detect the phenotypic activity of the efflux pump in S. aureus strains. Based on the results of this study, three strains were highly active among 30 strains, and did not exhibit fluorescence in all dilutions of ethidium bromide, which means they had strong efflux pumps. Among these three strains, one was MRSA. With the effect of 1/2 MIC of M. chamomilla extract on the strains with a strong efflux pumps, the two stains were reported as non-actives. M. chamomilla extract on the MRSA strain efflux pump was ineffective. (Figs. 2 and 3). Evaluation of 1/4 MIC of M. chamomilla extract had no effect on efflux pump activity. Khan et al. (2006) showed that the piperine, a plant alkaloid of the Piperaceae family, at a concentration of 25 mg/L reduced two-fold the MIC of S. aureus compared to the ciprofloxacin antibiotic. This decrease in MIC is due to the increase in the antibiotic concentration of ciprofloxacin in the bacterium, because of the inhibitory effect of the efflux pumps (28). Smith et al. used the Totarol isolated from the immature cones of Chamaecyparis nootkatensis to inhibit the NorA S. aureus pump. They used MIC analysis of ethidium bromide in combination with Totarol to evaluate pump inhibition and reported that the compound studied had antimicrobial activity and also inhibited the NorA pump (26).
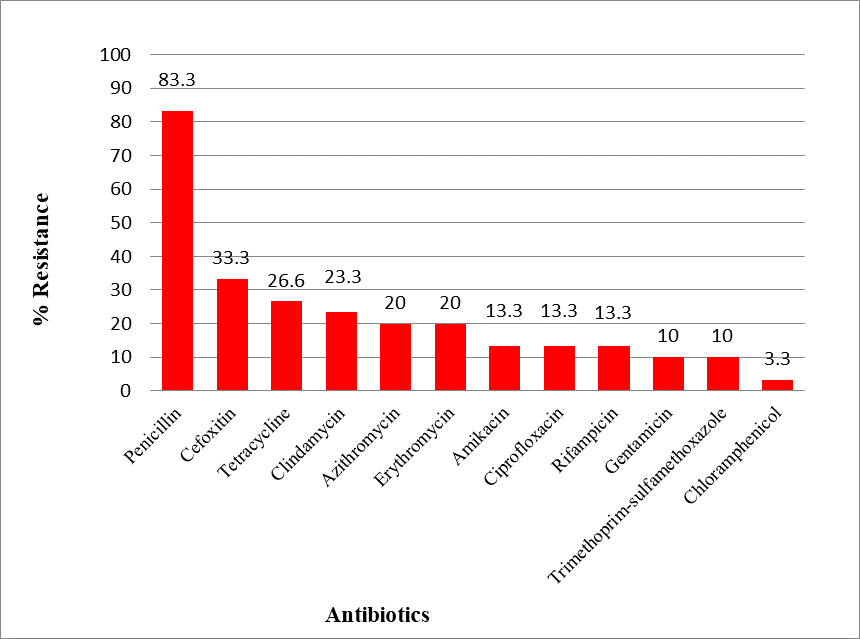
Figure 1. Antibiotic resistance patterns of Staphylococcus aureus strains

Figure 2. Fluorescent plates containing concentrations of 0.25 to 2.5 g/L of ethidium bromide and microbial suspension of Staphylococcus aureus strains: strains 13, 14 and 16 did not show fluorescent activity in all dilutions of ethidium bromide and became known as strong strains. Each growth line belongs to one isolate. Transparent culture lines indicate inactivation of the effluent pump and opaque cultivation lines indicate that the effluent pump system is active. Strain 14 was MRSA.
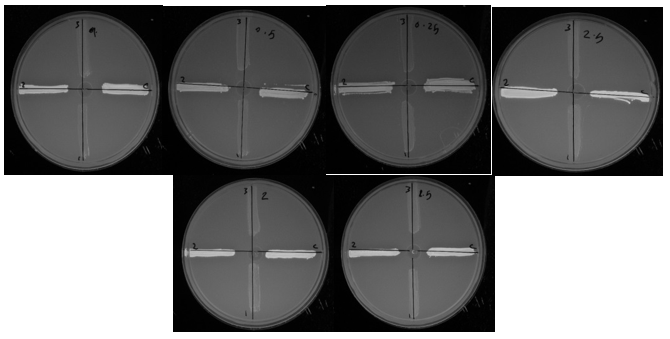
Figure 3. Fluorescent plate images containing ethidium bromide and microbial suspension with MIC concentration of 1/2 M. chamomilla extract of each strain: strains 13 and 16 showed fluorescent activity in all dilutions of ethidium bromide and were identified as inactive. MRSA strain 14 remained active.
There are major challenges to find new inhibitors. Considering the antimicrobial effects of plant compounds on inhibiting efflux pump, bacteria that were initially resistant to antibiotics can be sensitive later and, if this approach is successful in the future, it could be considered as an important alternative in the treatment of some infections caused by drug-resistant strains. It is also important to determine the expression level of these genes encoding efflux pumps among phenotypically active isolates, and that the role of synergy between efflux pumps and other antibiotic resistance mechanisms, to achieve this high resistance level, should not be ignored. Further studies are suggested to investigate the genotypic expression of efflux pumps in methicillin-resistant S. aureus strains.
The authors would like the staff of Microbiology Research Laboratory of Azad Islamic University, Varamin branch and also Mr. Omid Hosseini, the technician of the Research Laboratory of Shahid Beheshty University. The ethical code for this research was IR.IAU.VARAMIN.REC.1396.3.
The authors reported no conflict of interest.
Full-Text: (1821 Views)
Introduction
Staphylococcus aureus, in particular, methicillin resistant S. aureus (MRSA), is one of the main causes of hospital infections and community-acquired infections, with high morbidity and mortality rates worldwide (1). S. aureus infections cause difficult conditions to care and treat ulcers, damaged skin and soft tissue (2). MRSA are the principle cause of colonization and infection in the acute and chronic ulcerous lesions (4). Efflux Pump is one of the mechanisms of these bacteria in order to show antibiotic resistance (5). Many studies have investigated the inhibitory effect of some plant essential oils on efflux pumps in S. aureus strains (8-10). The simple way to evaluate the phenotypic effect of the efflux pump is the use of EtBr-Agar, which takes advantage agar plates and contains EtBr-increasing concentrations, which is known as the EtBr-Agar or cartwheel method (11, 12). Inside the plants, some compounds act in association with the antimicrobial agents, as they are responsible for disrupting the function of the efflux pumps in plant pathogens (7). Some plants have been involved in the treatment of skin diseases, and their effects have been proven like Matricaria chamomilla belongs to Asteracae family. This plant has antimicrobial and anti-inflammatory effects (13,14). The aim of this study was to investigate the effect of M. chamomilla alcoholic extract on phenotypes of MRSA strains isolated from skin lesions.
Materials and Methods
This study was performed on 30 samples of S. aureus collected from patients with skin lesions who referred to Shariati hospital in Tehran in the spring of 2017. The samples were characterized by different biochemical tests, including Gram stain, catalase, coagulase, DNase and mannitol fermentation, which led to identification and isolation. The antibiotics resistance of strains was assessed by disk diffusion method. To detect the phenotypic resistance of methicillin-resistant S. aureus, the susceptibility of the bacterial specimen to Cefoxitin disc (30 μg) in the Muller Hinton Culture medium was investigated. The M. chamomilla capitols were collected from Ardebil city and after taxonomic identification, they dried at 25°C in the shade and then powdered by the mechanical mill. The powder obtained from chamomilla capitols was mixed with 80% ethanol and then placed in a Soxhlet extractor. M. chamomilla alcoholic extract was prepared and MIC of it was obtained against MRSA strains by broth microdilution method. In order to investigate the activity of the efflux pumps phenotypically, all stains were evaluated by ethidium bromide containing agar using Cartwheel method (16). After identification of the strains with an activated efflux pump, the effect of M. chamomilla extract on the inhibitory activity of the pump was investigated. For this purpose, the dilutions 1/2 and 1/4 of the MIC of the extract were applied (according to the MIC level of each strain).
Results and Conclusion
According to the results, the highest antibiotic resistance of S. aureus strains was respectively related to penicillin (83.3%), cefoxitin (33.3%), while the lowest resistance belongs to chloramphenicol which reported (3.3 %). among 30 S. aureus strains, 10 strains (33.3%) were MRSA (Fig. 1). The results of this study and the comparison with other reports on the prevalence of methicillin-resistant strains indicate increased methicillin-resistant strains, one of the reasons of which has been due to the excessive use of antibiotics in recent years. MIC of M. chamomilla extract was tested for the studied strains and result obtained between 128-64 μg/ml. The antimicrobial effect of M. chamomilla extract and essential oil against a range of bacteria and fungi has been investigated and confirmed (20, 21).
In this study, ethidium bromide cartwheel assay was performed to detect the phenotypic activity of the efflux pump in S. aureus strains. Based on the results of this study, three strains were highly active among 30 strains, and did not exhibit fluorescence in all dilutions of ethidium bromide, which means they had strong efflux pumps. Among these three strains, one was MRSA. With the effect of 1/2 MIC of M. chamomilla extract on the strains with a strong efflux pumps, the two stains were reported as non-actives. M. chamomilla extract on the MRSA strain efflux pump was ineffective. (Figs. 2 and 3). Evaluation of 1/4 MIC of M. chamomilla extract had no effect on efflux pump activity. Khan et al. (2006) showed that the piperine, a plant alkaloid of the Piperaceae family, at a concentration of 25 mg/L reduced two-fold the MIC of S. aureus compared to the ciprofloxacin antibiotic. This decrease in MIC is due to the increase in the antibiotic concentration of ciprofloxacin in the bacterium, because of the inhibitory effect of the efflux pumps (28). Smith et al. used the Totarol isolated from the immature cones of Chamaecyparis nootkatensis to inhibit the NorA S. aureus pump. They used MIC analysis of ethidium bromide in combination with Totarol to evaluate pump inhibition and reported that the compound studied had antimicrobial activity and also inhibited the NorA pump (26).

Figure 1. Antibiotic resistance patterns of Staphylococcus aureus strains

Figure 2. Fluorescent plates containing concentrations of 0.25 to 2.5 g/L of ethidium bromide and microbial suspension of Staphylococcus aureus strains: strains 13, 14 and 16 did not show fluorescent activity in all dilutions of ethidium bromide and became known as strong strains. Each growth line belongs to one isolate. Transparent culture lines indicate inactivation of the effluent pump and opaque cultivation lines indicate that the effluent pump system is active. Strain 14 was MRSA.

Figure 3. Fluorescent plate images containing ethidium bromide and microbial suspension with MIC concentration of 1/2 M. chamomilla extract of each strain: strains 13 and 16 showed fluorescent activity in all dilutions of ethidium bromide and were identified as inactive. MRSA strain 14 remained active.
There are major challenges to find new inhibitors. Considering the antimicrobial effects of plant compounds on inhibiting efflux pump, bacteria that were initially resistant to antibiotics can be sensitive later and, if this approach is successful in the future, it could be considered as an important alternative in the treatment of some infections caused by drug-resistant strains. It is also important to determine the expression level of these genes encoding efflux pumps among phenotypically active isolates, and that the role of synergy between efflux pumps and other antibiotic resistance mechanisms, to achieve this high resistance level, should not be ignored. Further studies are suggested to investigate the genotypic expression of efflux pumps in methicillin-resistant S. aureus strains.
Acknowledgments
The authors would like the staff of Microbiology Research Laboratory of Azad Islamic University, Varamin branch and also Mr. Omid Hosseini, the technician of the Research Laboratory of Shahid Beheshty University. The ethical code for this research was IR.IAU.VARAMIN.REC.1396.3.
Conflict of Interest
The authors reported no conflict of interest.
Type of Study: Brief Original Article |
Subject:
Nosocomial infections
Received: 2019/07/29 | Accepted: 2019/11/22 | ePublished: 2019/11/22
Received: 2019/07/29 | Accepted: 2019/11/22 | ePublished: 2019/11/22
References
1. Imanifooladi AA, Sattari M, Peerayeh SN, Hassan ZM, Hossainidoust SR. Detection the Staphylococcus aureus producing enterotoxin isolated from skin infections in hospitalized patients. Pakistan journal of biological sciences. PJBS 2007; 10(3):502-5. [DOI:10.3923/pjbs.2007.502.505] [PMID]
2. Shahidi-Dadras M, Toossi P, Sarrafi-Rad N, Robati RM, Saeedi M, Kavand S. Staphylococcus aureus carriage in patients with psoriasis. Iranian Journal of Dermatology. 2009;12(1):1-3.
3. Jappe U. Superantigens and their association with dermatological inflammatory diseases: facts and hypotheses. Acta dermato-venereologica. 2000; 80(5):321-8. [DOI:10.1080/000155500459231] [PMID]
4. Tomi NS, Kränke B, Aberer E. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects. Journal of the American Academy of Dermatology. 2005; 1;53(1):67-72. [DOI:10.1016/j.jaad.2005.02.034] [PMID]
5. Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. Journal of Antimicrobial Chemotherapy. 2003; 1;51(1):9-11. [DOI:10.1093/jac/dkg050] [PMID]
6. Blanco P, Hernando-Amado S, Reales-Calderon J, Corona F, Lira F, Alcalde-Rico M, Bernardini A, Sanchez M, Martinez J. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms. 2016 4(1): [DOI:10.3390/microorganisms4010014] [PMID] [PMCID]
7. Gibbons S. Phytochemicals for bacterial resistance-strengths, weaknesses and opportunities. Planta medica. 2008; 74(06):594-602. [DOI:10.1055/s-2008-1074518] [PMID]
8. Stavri M, Piddock LJ, Gibbons S. Bacterial efflux pump inhibitors from natural sources. Journal of antimicrobial chemotherapy. 2006; 4;59(6):1247-60. [DOI:10.1093/jac/dkl460] [PMID]
9. Gibbons S, Oluwatuyi M, Kaatz GW. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 2003; 1;51(1):13-7. [DOI:10.1093/jac/dkg044] [PMID]
10. Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrobial agents and chemotherapy. 2002; 1;46(10):3133-41. [DOI:10.1128/AAC.46.10.3133-3141.2002] [PMID] [PMCID]
11. Pumps E, Cost F, RamA B. Efflux pumps of gram-negative bacteria: genetic responses to stress and the modulation of their activity by pH, inhibitors, and phenothiazines. Advances in enzymology and related areas of molecular biology. 2011; 15;238:61. [DOI:10.1002/9780470920541.ch2]
12. Amaral L, Cerca P, Spengler G, Machado L, Martins A, Couto I, et al. Ethidium bromide efflux by Salmonella: modulation by metabolic energy, pH, ions and phenothiazines. International journal of antimicrobial agents. 2011; 38(2): 140-5. [DOI:10.1016/j.ijantimicag.2011.03.014] [PMID]
13. Abdoul-Latif FM, Mohamed N, Edou P, Ali AA, Djama SO, Obame LC, Bassolé IH, Dicko MH. Antimicrobial and antioxidant activities of essential oil and methanol extract of Matricaria chamomilla L. from Djibouti. Journal of Medicinal Plants Research. 2011; 4;5(9):1512-7.
14. Charousaei F, Dabirian A, Mojab F. Using chamomile solution or a 1% topical hydrocortisone ointment in the management of peristomal skin lesions in colostomy patients: results of a controlled clinical study. Ostomy-Wound Management. 2011; 1;57(5):28.
15. Sharifi A, Mohammadzadeh A, Mahmoodi P, Sasanian N. Inhibitory Effect of Thymus daenensis Essential Oil on Staphylococcus aureus NorA Efflux Pump. J Adv Med Biomed Res. 2016; 24 (105) :67-77
16. Martins M, McCusker MP, Viveiros M, Couto I, Fanning S, Pagès JM, Amaral L. A simple method for assessment of MDR bacteria for over-expressed efflux pumps. The open microbiology journal. 2013; 7:72. [DOI:10.2174/1874285801307010072] [PMID] [PMCID]
17. Movagharnezhad M, khataminezhad M R. Identification and Characterization of Staphylococcus Aureus Methicillin and Vancomycin Resistance From Patients in Sari and Ghaemshahr Injuries and Burn Hospitals in 2015. Iran J Med Microbiol. 2018; 12 (3) :160-168 [DOI:10.30699/ijmm.12.3.160]
18. Fagheei-Aghmiyuni Z, Khorshidi A, Soori T, Moniri R, Mousavi S G A. Antibiotic susceptibility pattern and the prevalence of Staphylococcus aureus isolated from skin and soft tissue in Tehran Razi skin hospital (2014-15). Feyz. 2017; 21 (2) :188-196
19. Motamedi H, Rahmat Abadi SS, Moosavian SM, Torabi M. The Association of PantonValentine leukocidin and mecA Genes in Methicillin-Resistant Staphylococcus aureus Isolates From Patients Referred to Educational Hospitals in Ahvaz, Iran. Jundishapur J Microbiol 2015; 8(8). [DOI:10.5812/jjm.22021v2] [PMID] [PMCID]
20. Jarrahi M, Vafaei AA, Taherian AA, Miladi H, Rashidi Pour A. Evaluation of topical Matricaria chamomilla extract activity on linear incisional wound healing in albino rats. Natural product research. 2010; 10;24(8):697-702. [DOI:10.1080/14786410701654875] [PMID]
21. Roby MH, Sarhan MA, Selim KA, Khalel KI. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Industrial crops and products. 2013; 1(44):437-45. [DOI:10.1016/j.indcrop.2012.10.012]
22. Piddock LJ, Garvey MI, Rahman MM, Gibbons S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. Journal of Antimicrobial Chemotherapy. 2010; 19; 65(6):1215-23. [DOI:10.1093/jac/dkq079] [PMID]
23. Haddadi Zahmatkesh M A, Laripoor M, Mirzaie A, Ashrafi F. Prevalence of norA and norB efflux pump genes in clinical isolates of Staphylococcus aureus and their contribution in ciprofloxacin resistance. Iran J Med Microbiol. 2016; 10 (5) :20-30
24. Martins M, Viveiros M, Couto I, Costa SS, Pacheco T, Fanning S, Pages JM, Amaral L. Identification of efflux pump-mediated multidrug-resistant bacteria by the ethidium bromide-agar cartwheel method. in vivo. 2011; 1;25(2):171-8.
25. Patel D, Kosmidis C, Seo SM, Kaatz GW. Ethidium bromide MIC screening for enhanced efflux pump gene expression or efflux activity in Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2010; 1;54(12):5070-3. [DOI:10.1128/AAC.01058-10] [PMID] [PMCID]
26. Smith EC, Kaatz GW, Seo SM, Wareham N, Williamson EM, Gibbons S. The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2007; 1;51(12):4480-3. [DOI:10.1128/AAC.00216-07] [PMID] [PMCID]
27. Sana M, Jameel H, Rahman M. Miracle remedy: Inhibition of bacterial efflux pumps by natural products. Journal of Infectious Diseases & Therapy. 2015; 30. [DOI:10.4172/2332-0877.1000213]
28. Khan IA, Mirza ZM, Kumar A, Verma V, Qazi GN. Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2006; 1;50(2):810-2. [DOI:10.1128/AAC.50.2.810-812.2006] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







