BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2519-en.html
2- Department of Pharmacochemistry, Faculty of Pharmacy, Universitas Muhammadiyah Purwokerto, Purwokerto, Indonesia
3- Department of Pharmacy, Faculty of Pharmacy, Universitas Muhammadiyah Banjarmasin, Banjarmasin, Indonesia
Concerns about food safety due to microbial contamination have increased. Many outbreaks due to microbial contamination of fruit juices have been reported worldwide (1, 2). Fruit juice can be contaminated with pathogenic microorganisms through various routes, such as using contaminated raw materials during harvest, adding contaminated water to the juice, or the water used for washing the equipment (3, 4). Unpasteurized fruit juices have been reported to be contaminated with pathogenic bacteria such as E. coli, Salmonella sp., Shigella sp., Staphylococcus aureus, Campylobacter jejuni, and Cryptosporidium parvum (5-7). Research conducted in Vidarbha, India, showed that of 115 juice samples, 17.14% were contaminated with Shigella (8). Shigella dysenteriae was discovered in orange and other juice samples in Guadalajara (9). Aerobic plate counts of ≥ 5.0 log CFU/mL were found in 31% of the juice samples. Shigella flexneri and Shigella boydii were also found in orange juices (9). In Mekelle, Ethiopia, the prevalence of Shigella spp. was significant in ready-to-eat fruit juices and salads (10). These data could indicate unsanitary raw materials or the juice serving procedure. Shigella dysenteriae is the leading cause of human shigellosis or bacillary diarrhea (11).
Although some fruit juices such as orange juice have acidic pH (4.19-4.50), which is an essential barrier to bacterial growth, some pathogenic bacteria can survive under these conditions (12). Therefore, a decontamination stage is needed in orange juices.
Juice microbial contamination is usually controlled using heat treatment, such as pasteurization or sterilization (13, 14). However, the methods can reduce the bioactive content of the fruit juice. A study indicated that anthocyanins in pomegranate juice were dramatically reduced from 332.31 ± 5.21 to 263.84 ± 6.84 (mg/L cyanidin-3-glucoside) after heat treatment at 85ºC for 10 min (15). Furthermore, several non-thermal methods have been proposed, including ultrasonication, cold plasma, supercritical, irradiation (gamma rays, X-rays, IR, high-speed electrons), pulse electric field, high hydrostatic pressure, pulse ultraviolet, and ozone (16, 17). Ascorbic acid content was significantly reduced during ultrasonication due to extreme physical conditions and sonolysis (18, 19), whereas ozone treatment affected turbidity, color intensity, and acid content of orange juice (20). Furthermore, previous research reported that several non-thermal methods were quite effective in preserving the nutritional value of the juice but were ineffective in inactivating pectin methyl esterase and polyphenol oxidase, both of which play role in maintaining the color of the juice, causing pale appearance (21). As a result, the need for more effective antibacterial technologies, such as photodynamic inactivation (PDI), is urgent and crucial.
PDI involves a combination of photosensitizers, light, and oxygen. Photodynamics has been used in medical field to treat tumors, cancers, and infections, and it is known as photodynamic therapy (PDT). In line with this, PDI was developed to inactivate pathogenic microorganism cells. PDI destroys microorganisms by inducing oxidative stress in cells (22, 23). The PDI mechanism is based on formation of reactive oxygen species (ROS), including singlet oxygen, which triggers oxidation reactions in microbial cells with the main targets being cell membrane proteins and lipids, as well as DNA (24).
Previous research reported that PDI effectively inactivated pathogenic microorganisms in fruit juice. The researchers from the University of Freiburg used natural photosensitizers in the mother juice of bilberry, pomegranate, and chokeberry to kill Streptococcus mutans and Streptococcus sobrinus (25). Modification of curcumin natural photosensitizer through formation of a complex of biotin-modified β-cyclodextrin and curcumin (Biotin-CD@Cur) has proved to increase the ability of curcumin to kill Listeria monocytogenes and Staphylococcus aureus in freshly squeezed orange juice (26). Meanwhile, PDI has been proven to be able to reduce the number of pathogenic bacteria, E. coli, L. monocytogenes, and S. typhimurium in tomato juice (27).
The potential of PDI in decontamination of orange juice is significant. This technique superiority is mainly because many traditional food preservation techniques can reduce nutritional components or change the product sensory properties. A previous research using curcumin-based system showed that PDI effectively preserves the nutritional content of freshly squeezed orange juice, including essential vitamins and antioxidants, while ensuring microbial safety (26). The taste, pH, color, and lycopene content of tomato juice treated with PDI showed no significant changes after treatment (27).
By selecting an appropriate photosensitizer, PDI can be effectively applied. Unlike PDT, PDI can use compounds with absorption in the “soret” area (around 400 nm) or the visible area up to 550 nm. The food dye erythrosine B (2-[6-hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl] benzoic acid) could be a key player in this process. The unique potential of erythrosine B in PDI process is intriguing aspect of this research. It sparks interest and curiosity in its application to overcome pathogenic bacterial contamination of Shigella dysenteriae in orange juice.
Shigella dysenteriae isolates from the Faculty of Medicine, Universitas Jenderal Soedirman were used as test organisms. The isolate was obtained from patients in the hospital. Morphological and biochemical tests were conducted to identify the bacteria. Two PDT green lights (PDT Omega Light Therapy, λ532 nm/LG) and two LED polychromatic bulbs (Krisbow, 1800 lumen/LP) were adopted for irradiation. The power of light was measured using a lux meter (Krisbow KW06-291). The distance of the lamp to the crystallizing dish was 27 cm. Tryptic Soy Agar (TSA) and Tryptic Soy Broth (TSB) were obtained from Himedia, India.
Preparation Stage
The preparation stage was meticulously carried out to ensure the reliability of the experiments. TSA and TSB were used as bacterial growth media. TSA (2 gr in 50 mL distilled water) and TSB (0.9 gr in 30 mL distilled water) were heated until completely dissolved. They were both sterilized by autoclaving.
The pure culture of Shigella dysenteriae was carefully handled and streaked onto TSA media. It was then incubated for 24 hr at 37°C. Next, the bacteria (1-2 inoculating loop) were transferred to TSB (5 mL) in a test tube, and incubated (37oC, 24 hr). An inoculum of 1.5 x 108 CFU/mL (absorbance 0.08-0.1, 600 nm) was prepared for further tests. The number of bacterial colonies was monitored by comparing the turbidity of the inoculum with standard solution 0.5 Mc Farland.
Sterile erythrosine B (500 µM in distilled water) was prepared and protected from exposure to light. A portion of the solution was measured using UV Vis spectrophotometry (Shimadzu 1240) to obtain the absorption spectrum.
Fresh orange fruit was obtained from the local market in Purwokerto, Indonesia. The fruit was squeezed and filtered. The color, pH, and temperature of orange juice were observed before and after treatment.
Photodynamic Inactivation Treatment
This treatment involved the addition of erythrosine B and light (+E+L). Before treatment, orange juice (50 mL) was combined with bacterial suspension (0.75 mL) on a sterile crystallizing dish. Then 0.5 mL erythrosine B (500 µM) was added, and the mixture was homogenized. The dish was illuminated with LED PDT green lights at various illumination times (10, 20, and 30 min). A similar treatment was performed by irradiation on samples with a polychromatic LED bulb.
Bacterial colony counts were carried out using colony counter method. Samples were diluted in different stages from 10-1 to 10-4 using buffered peptone water, then, transferred to TSA media in a petri dish, incubated for 24 hr at 37oC, and the number of colonies formed was counted.
Erythrosine B antibacterial activity test (dark toxicity)
This treatment excludes the irradiation stage (+E-L). The treatment stages and the amounts of components included were similar to the photodynamic inactivation treatment step. During treatment, the sample was kept in dark.
Antibacterial activity by irradiation
This treatment only involved radiation without inclusion of photosensitizer erythrosine B (-E+L). The steps were similar to those of the photodynamic inactivation treatment. All the experiments were evaluated in triplicate. Data was displayed as a percentage of bacterial viability.
The addition of erythrosine B and irradiation showed a subtle impact on pH and temperature of the solution. The rise in temperature post-treatment is due to the heat emitted by the lamp used. Notably, LP lamps outperform LG lamps in heat generation, leading to a temperature surge of 5.2-6.4% (vs. LG 2.0-2.8%).
Under our meticulously controlled experimental conditions, we observed that erythrosine has a slight impact on orange juice pH. Addition of erythrosine B led to a pH change of 0.7%. After a 30-min irradiation, the pH showed an increase between 1.0-1.8% (Table 1).

Figure 1. The colour of orange juice before (left) and after (right) adding erythrosine B
Table 1. Physicochemical characteristics of orange juice before and after treatment for 30 min.
| Treatment | Before treatment | After treatment | ||||
| pH | Temp. (oC) | Colour | pH | Temp. (oC) | Colour | |
| -E+LG | 3.96±0 | 25.2±0.1 | DO | 3.96±0 | 25.7±0* | DO |
| -E+LP | 3.96±0.01 | 26.7±0.1 | DO | 3.96±0 | 28.4±0* | DO |
| +E-L | 3.99±0 | 25.5±0.2 | DO | 4.02±0* | 25.8±0.1 | DO |
| +E+LG | 3.99±0 | 25.0±0.1 | DO | 4.06±0.01* | 25.7±0* | DO |
| +E+LP | 3.99±0 | 26.9±0 | DO | 4.03±0.01* | 28.3±0.1* | DO |
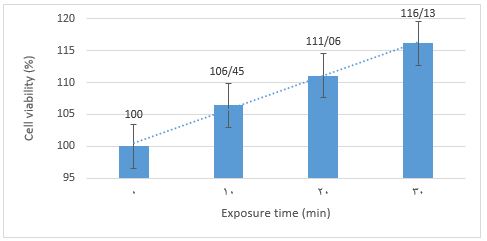
The antibacterial activity test using light without a photosensitizer was conducted to determine if it could reduce the number of Shigella dysenteriae in orange juice. The juice containing bacteria was exposed to LG and LP lights for 10, 20, and 30 min with light intensity, as shown in Table 2. Bacterial observations were made in a petri dish at 10000x dilution. The results showed that LG and LP light sources did not inhibit the growth of the bacteria (Figure 2). However, it was noted that bacterial growth increased significantly when exposed to LP for 30 min, reaching 12.52% compared to the initial bacteria count.
Table 2. The intensity of PDT green lights (LG) and LED bulb polychromatic (LP)
| No | Type of light | Intensity | |
| lux | mW/cm2 | ||
| 1. | Blue light (λ 423 nm) | 2270 | 0.331 |
| 2. | Green light (λ 532 nm) | 1388 | 0.203 |
| 3. | Red light (λ 640 nm) | 759 | 0.111 |
| 4. | LED bulb polychromatic | 4990 | 0.730 |
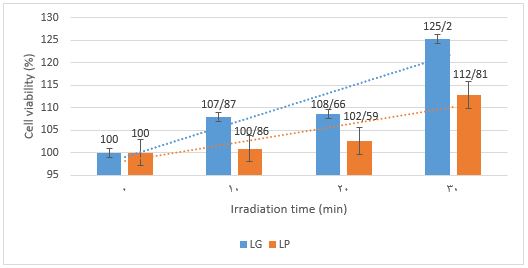
Figure 2. Viability percentages of Shigella dysenteriae in treatment without addition of erythrosine B using LG (-E+LG) and LP (-E+LP) irradiation. LG=PDT green lights. LP=LED bulb polychromatic.
Erythrosin B antibacterial test without irradiation, known as dark toxicity, was performed to evaluate whether the compound could inhibit or was toxic to Shigella dysenteriae. Orange juice containing bacteria and erythrosine B was kept in a dark room to avoid light as activator of erythrosine B. The results demonstrated that erythrosine B was neither poisonous nor capable of inhibiting bacterial growth under the test conditions (Figure 3). Bacterial growth continued to increase as exposure time increased.
Our study on PDI using erythrosine B has yielded promising results. The combination of irradiation and erythrosine B in PDI significantly reduced the viability of Shigella dysenteriae (Figure 4). This suggests that PDI using erythrosine B could be a potential breakthrough, offering a favorable and promising alternative method for controlling spoilage and pathogenic microorganisms, especially Shigella dysenteriae, in orange juices.
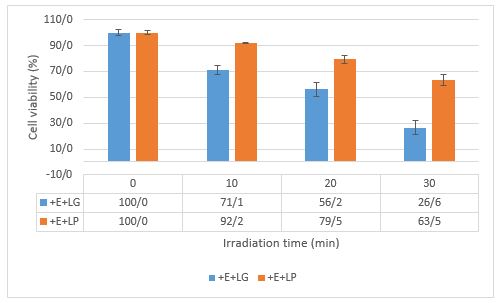
Figure 4. The fffect of irradiation time on survival of Shigella dysenteriae by PDI. The concentration of erythrosine B was 5 μM with LG intensity at 1388 lux (0.203 mW/cm2) and LP intensity at 4990 lux (0.730 mW/cm2).
The photosensitizer transforms from the ground state to the excited singlet state and then reaches the excited triplet state (3PS*). After reaching that state, two types of reactions can occur. In type I reactions, photosensitizers react via electron transfer with biomolecules, such as lipids, proteins, and amino acids, to produce superoxide anion radicals (O2•−) and hydroxyl radicals (•OH). The O2•− undergoes dismutation to become H2O2. In type II reactions, the photosensitizer produces singlet oxygen (1O2) through direct energy transfer to oxygen molecules. Like hydroxyl radicals, 1O2 is very reactive. These two types of reactions compete with each other, and type II reactions are believed to be the primary mechanism of PDT in the presence of oxygen (30). Excessive ROS production in bacteria can cause bacterial death through a variety of mechanisms, including damaging cellular components (e.g., bacterial lipids, proteins, and DNA) (31), increasing cell membrane permeability (32), disrupting normal physiological metabolism (32), and even accelerating gene mutations (33), which eventually result in cell death (34).
In a recent study, PDI was used to inactivate Shigella dysenteriae using erythrosine B. The involvement of light, photosensitizers, and oxygen in the PDI process was confirmed to be responsible for this inhibition.
The confirmation was achieved through treatments without erythrosine B (Figure 2) and without light (Figure 3), which resulted in inability to inhibit bacterial growth. However, negative control (-E-L) was not used in this investigation for as the environmental conditions of orange juice (pH and temperature) are known to support the growth of microbes optimally. A foodborne bacterium, Shigella dysenteriae, may live in several environments, including fruit juices. The lowest and the maximum temperatures at which these bacteria may grow are 6-7oC and 45-47oC, respectively. Shigella dysenteriae can live for up to six days in orange juice. This bacterium grows at a minimum pH of 4.8-5.0 and a maximum of 9.3. It can even grow well in the absence of oxygen (35). Thus, it can be assumed that negative control (-E-L) does not provide any relevant additional information and can be omitted with the hope that this study can focus more on comparing the parameters of light source type and irradiation time variations and can save time, materials, and energy.
Erythrosine B, an FDA-approved food colorant (36), is not only suitable as a photosensitizer but also a safe choice for food use. The natural color of the fruit, like tomato, orange, and strawberry, remains unaltered by erythrosine B and may even become more intense. The color of the juice is a critical factor in consumer approval (37). Our results showed that the color of the solution did not change, aligning with previous research. This consistency further validates our findings, as previous research also showed no significant difference in the color of tomato juice treated with erythrosine B (100 μM) mediated PDI for 15 min compared to the control (27).
Two light types; LG and LP were evaluated as light sources in this experiment. The green light in the LG lamp is a complementary color to red and correlates with the red dye erythrosine B. Therefore, this lamp is suitable for exciting the erythrosine B solution. This finding is not standalone but aligns with earlier studies that reported erythrosine B light absorption range between 450 and 550 nm (38). The absorption spectrum of erythrosine B, with a maximum absorption of 526 nm (Figure 5), further supports our research. On the other hand, although the intensity of LP light is higher than LG, not all polychromatic wavelengths are used to stimulate erythrosine B (23, 24), thus, the effect is not as good as LG.
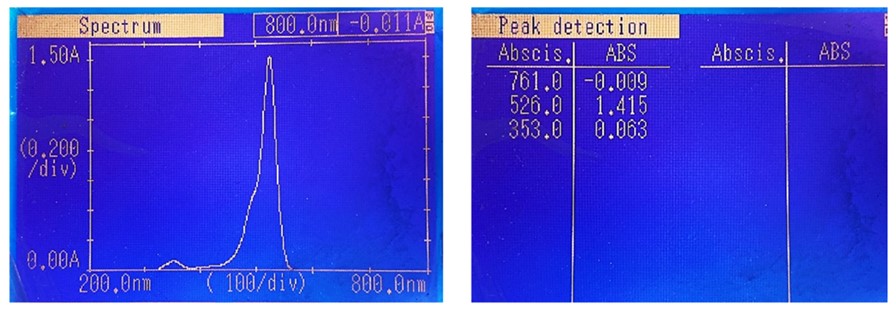
Figure 5. The absorption spectrum of 0.015% erythrosine B in distilled water shows maximum absorption at 526 nm wavelength.
In this study, irradiation for 30 min could inhibit bacterial growth for up to 73.4%. The performance of erythrosine B as a photosensitizer in PDI was in line with previous research, which observed inhibition in growth of E. coli, S. typhimurium, and L. monocytogenes, resulting in decreases to 6.77, 2.74, and 6.43 log CFU/mL, respectively (27).
Meanwhile, another research group used PDI to inactivate Alicyclobacillus acidoterrestris in orange juice (11). The orange juice industry was highly concerned about A. acidoterrestris because of its resistance to heat, chemicals, and deterioration. Interestingly, the phenothiazinium dye new methylene blue (NMB) and the tetracationic porphyrin (Tetra-Py+-Me) used as photosensitizers were unable to inhibit A. acidoterrestris without adding KI. After being exposed to white light for 10 hr (140 mW/cm2), KI can help to inactivate 5 log CFU/mL of A. acidoterrestris spores. These authors claim that the action of KI with 1O2 allows for the creation of longer-lived reactive species like free iodine and triiodide, which is the cause of this potentiation. In our study, it seems that the effectiveness of erythrosine B in producing ROS was better than Tetra-Py+-Me and NMB, thus, it can be used to inactivate Shigella dysenteriae without addition of KI.
The intricacy of the matrix, which influences turbidity and light penetration in the solution, was linked to the limitations of PDI application in inhibiting pathogenic microorganisms in fruit juice. This research highlights the need for further investigation into the role of matrix complexity in PDI performance. For instance, previous research has shown that apple juice PDI performance is poorer than grape juice due to its higher turbidity, which restricts light penetration (11). Another limitation of this study is that the method effectiveness must be compared with other methods, such as disinfectants or thermal methods. Further research using comparative methods could be an interesting step to provide more concrete results regarding the effectiveness of PDI using erythrosine B to inhibit Shigella dysenteriae in orange juice.
ROS, especially singlet oxygen, plays an important role in PDI. PDI has gained advantages in its application compared to the thermal method as it maintains a balance between microbial decontamination and sensory attributes (color, flavor, or texture) of treated food. It does not adversely induce changes in physico-chemical properties and nutritional qualities (40-43). For its food application, there is concern about the interaction of singlet oxygen with important components in food to produce undesirable by-products. The short lifetime of singlet oxygen (50 to 700 μs, depending upon the solvent system of food) and the presence of quencher compounds in food such as tocopherol, carotenoids, ascorbic acid, and quercetin minimize the side effects of singlet oxygen (44). However, validating the method and ensuring no residual ROS in the juice is crucial. Its application must be accompanied by approval of the relevant authorities.
TThe study findings suggest that Shigella dysenteriae in orange juice can be effectively inactivated by erythrosine B-mediated PDI without compromising the juice quality. The type of light and its intensity determine the effectiveness of this method.
Nothing to declare.
Ethical Considerations
None.
Authors’ Contributions
ADD and PIU designed the topic, supervised the work, and wrote the manuscript. AAT, IZ: collected and analyzed data. All authors read and approved the final version of the manuscript.
Conflicts of Interest
Received: 2024/11/11 | Accepted: 2025/01/15 | ePublished: 2025/01/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |