BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2472-en.html


 , Samin Aboulhassanzadeh2
, Samin Aboulhassanzadeh2 

 , Hamed Aghazadeh3
, Hamed Aghazadeh3 

 , Zahra Nikkhooy4
, Zahra Nikkhooy4 

 , Behboud Jafari5
, Behboud Jafari5 


2- Mérieux NutriSciences - Global, Advanced laboratory testing, Newbridge, Ireland & Research Center for Pharmaceutical Nanotechnology (RCPN), Tabriz University of Medical Sciences, Tabriz, Iran
3- Research Center for Pharmaceutical Nanotechnology (RCPN), Tabriz University of Medical Sciences, Tabriz, Iran & School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
4- Department of Biology, Faculty of Science, Shahid Chamran University, Ahvaz, Iran
5- Department of Microbiology, Ahar Branch, Islamic Azad University, Ahar, Iran
Escherichia coli (E. coli) is a Gram-negative bacterium with certain serotypes, such as O157:H7, that can cause foodborne illness and diarrhea in human (1). This bacterium is the most common cause of urinary tract infections, accounting for approximately 90% of the urinary infections in women (2). At the same time, this important pathogenic microorganism has a variety of uses in industry and medicine (1). Based on the toxins production there are different types of E. coli including Shiga toxigen-producing E. coli (STEC), verotoxin-producing E. coli (VTEC), and enterohemorrhagic E. coli (EHEC). including O157-, CFT073-, and K-12-specific (2). One of the highly important serotypes of EHEC is E. coli O157:H7, which is transmitted to human through food and causes dangerous complications such as bloody colitis (3). E. coli expresses the Hfq gene as regulator of the virulence traits (4).
The Hfq in E. coli is a very conserved protein modulating the degradation of target RNAs, facilitating the sRNA-mRNA pairing, and stabilizing the sRNAs (3). Recently, scientists focused on recruiting the microbiome as a novel therapeutic for the E. coli-related infections (3, 5).
Bifidobacterium is the most important anaerobic Gram-positive, highly pleomorphic, and non-spore-forming probiotic bacteria in the normal flora of intestine (6). This bacterium has beneficial effects including the production of essential vitamins and increased digestibility of proteins as well as preventing intestinal infections and diarrhea, reducing radiotherapy-induced disorders, treating certain brain lesions, lowering blood cholesterol levels, reducing cancer-related compounds, and immunological effects by strengthening the immune system or reducing the lactose intolerance effects (7, 8).
In 2008, Carey et al (9) reported the impact of probiotics on reducing the production of Shiga toxin type 2 (STX2) in E. coli O157:H7. Ahmadizadeh et al (10), in 2018, documented the effect of probiotics in reducing the expression of verotoxin genes STX1 and STX2. Takahashi et al (11), in 2004 reduced the production of Shiga toxins STX1 and STX2 in E. coli-infected mice using the probiotic bacteria Clostridium butyricum. Asahara et al (12) reported in 2004 that probiotics could reduce the mortality from STEC O157:H7 in mice. Wan et al (13), in 2019 suggested using probiotics to control foodborne bacteria and toxin-producing contaminants, proposing that their addition can control these factors. Cordonnier et al (14) suggested that probiotics and enterohemorrhagic Escherichia coli has an effective strategy against a deadly enemy. Ku et al (15) recently published an article stating that probiotic consumption is very safe for humans and can be used to combat the microbial infections, especially toxin-producing microbes.
The aim of this research was to study the effect of co-cultivation of the Bifidobacterium Longum (B. Longum) as a probiotic bacterium with pathogenic E. coli O157:H7 on the expression level of the virulence Hfq gene and its growth rate.
E. coli O157:H7 bacteria were obtained from the Collection Center of Infectious and Industrial Microorganisms, (Iran) and cultured in liquid LB medium for 16 hours at 37°C. The cultured sample was then stocked in 25% glycerol solution and stored at -70°C.
The culture medium, necessary chemicals, and solutions used in this research include: DEPC, diethyl pyrocarbonate; dNTPs, deoxynucleotide triphosphate monomers; EDTA, ethylenediaminetetraacetic acid; HCL, hydrochloric acid; KCl, potassium chloride; KH2PO4, potassium dihydrogen phosphate; Na2HPO4, disodium hydrogen phosphate; NaCl, sodium chloride; NaHCO3, sodium bicarbonate; NaOH, sodium hydroxide; and phosphate buffer saline (PBS).
2.2. Methods
2.2.1. E. coli Hfq Gene PCR Amplification
The salting-out method was employed for the DNA extraction of E. coli. Briefly, the cultured bacteria were centrifuged at 10,000 rpm for 5 min and the supernatant was mixed with 850 μl pre-heated lysing buffer at 65°C. The samples were then incubated at 65°C for 30 min and 5-6 min, respectively. Then, 700 μl chloroform-isoamyl alcohol solution was added to the samples and centrifuged at 10,000 rpm for 10 min. The isolated DNA was then washed with cold isopropanol (´1.5 volume of the sample), stored overnight at -70°C, centrifuged at 10,000 rpm for 5 min, and dried at room temperature. Finally, DNA samples were dissolved in 50 μl DNase-free deionized distilled water and stored until further evaluations.
Then, polymerase chain reaction (PCR) was performed mixing 12.5 µl PCR master mix (2x), 100 ng DNA, specific primers, and H2O up to 25 µl volume. The PCR reaction cycling involved initial denaturation at 95°C for 4 min, followed by 32 cycles of denaturation at 94°C for 1 min, primer annealing at 57°C for 80 sec, extension at 72°C for 1 min, and a final extension step at 72°C for 10 min. The amplified DNA samples were also loaded on 1% agarose gel with loading buffer in a 6:1 ratio and evaluated using SYBR Green dye and UV light.
2.2.2. Co-culturing E. coli with Bifidobacterium
Initially, E. coli was cultured in Mueller-Hinton Agar (MHA) under aerobic conditions. Simultaneously, Bifidobacterium was cultured in MRS (De Man, Rogosa, and Sharpe) under anaerobic conditions completely deoxygenating by purging with nitrogen gas and autoclaved. Then, different concentrations of this bacterium were used to treat E. coli at concentration of 0.5 McFarland. To investigate the expression of the virulence gene, the cultures were collected and centrifuged at various time intervals. The range of concentrations were obtained from preliminary experiments (serial dilutions) and literature (7, 8).
2.2.3. The Hfq Expression Levels Evaluation
For RNA extraction, 500 μl Trizol solution was added to the culture medium and incubated at 45°C for 90 min. After that, 200 μl chloroform was added, incubated at room temperature for 5 min, and centrifuged at 12,000 rpm for 10 min. The supernatant was mixed with 2.5x cold isopropanol and stored at -70°C overnight. Then, samples were centrifuged at 12,000 rpm for 10 min and dried. Then, 20 μl DEPC-treated water was added to the RNA pellet and incubated for 10 min at room temperature. The OD was measured in ng/ml using a NanoDrop instrument and the samples were stored at -70°C.
For the reverse transcription and cDNA synthesis, a mixture of 500 ng total RNA, 0.2 μM random hexamer primer, 1 μl dNTP, and DEPC-treated water were incubated at 65°C for 5 min. Then, 5 units of MMLV enzyme, 1x buffer for MMLV RT, and 1 U/μl RNase inhibitor were added and incubated at 25°C for 10 min, 42°C for 60 min, and 72°C for 10 min.
Negative control (RNA sample without reverse transcriptase) and positive control (known concentration of target RNA) were used during RNA extraction and cDNA synthesis.
In real-time PCR reactions, cDNA, master mix, forward and reverse primers (0.2 μM/each) and DEPC-treated water were mixed and the cycling was performed as mentioned before. The primers were designed using the oligo7 program, blasted through the NCBI website (www.ncbi.nlm.nih.gov) and synthesized by Takapouzist Company, Iran. The 16S ribosomal RNA was used as an internal control for data normalization. The specifications of the used primenrs are shown in Table 1.
Table 1. Specifications of primers used in real-time PCR method
| Annealing temperature | Primer sequence | The length of the pieces | Gene |
| 51 °C |
Forward:5’- ATAAATCGCCATTCGTTGACTA-3’ Reverse:5’- AGAACGCCCACTGAGATCATC-3’ |
180 bp | Hfq |
| 53 °C | Forward: 5’-ACTCTGTTATTAGGGAAGAA-3’ Reverse:5’- AACGCTTGCCACCTACGTAT-3’ |
90 bp | 16s rDNA |
The cycle threshold (CT) values were used to evaluate the expression level of Hfq gene. The obtained data were normalized to 16s rDNA housekeeping gene and the gene expression ratio was calculated using equation provided by Asahara (12):
The relative expression ratio of a target gene is computed, based on its real-time PCR efficiencies (E) and the crossing point (CP) difference (Δ) of an unknown sample versus a control (ΔCPcontrol – sample).
The data analysis was performed via SPSS software. Data normality was checked via the Shapiro-Wilk test and due to the normal distribution, the comparison between two groups was performed via independent t-test. The P-value less than 0.05 was considered significant.
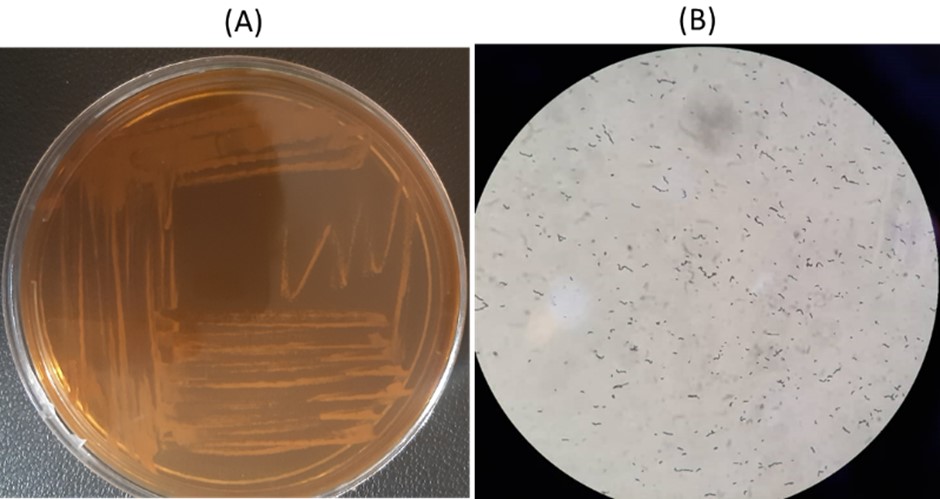
The culture of B. Longum was conducted in anaerobic conditions and in MRS shown in Figure 1.
3.2. Co-Culture of E. coli with B. Longum
The growth of E. coli alone and in the presence of B. Longum was conducted at 37°C and aerobic conditions over 20 hours using 1 ml of bacteria in the cultured medium. Following each corresponding incubation interval, the culture was collected, centrifuged at 300 rpm and the bacterial pellet was resuspended in 1 ml PBS and growth rate was measured using Nanodrop at 600 nm wavelength in treplicates (Figure 2). B. Longum showed the capability to inhibit the growth of E. coli significantly, achieving nearly a two-fold reduction.
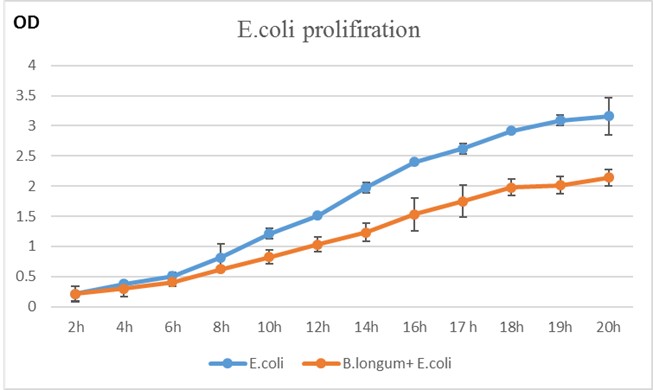
Figure 2. E. coli proliferation rates alone and in co-culture with B. Longum based on OD at 600 nm at 2, 4, 6, 8, 10, 12,14,16, 17, 18, 19, and 20 hours.
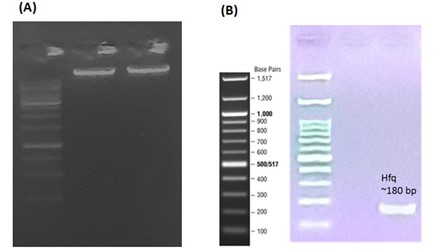
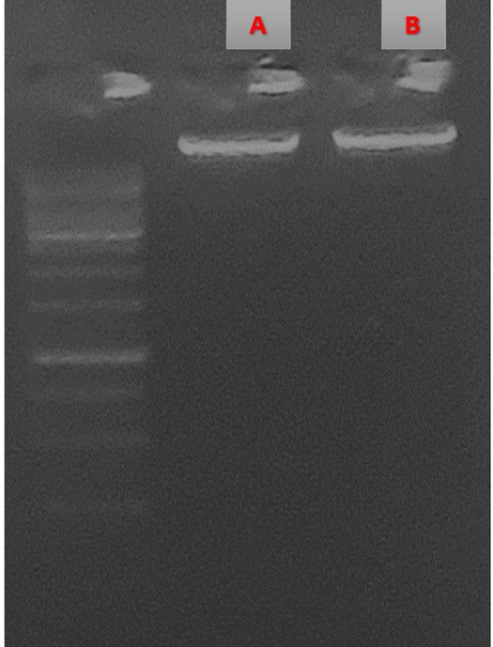
The presence of the Hfq gene in E. coli was confirmed for further comparative gene expression analysis. The DNA extraction from E. coli bacteria and the confirmation of the presence of the Hfq gene were carried out before and after DNA amplification of E. coli O157:H7 (Figure 3). After performing PCR, a distinct amplification band for the Hfq gene of approximately 180 bp was observed as expected.
To evaluate the changes in the expression of the Hfq gene, total RNA extracted from E. coli bacteria alone or in co-culture with B. Longum was evaluated.
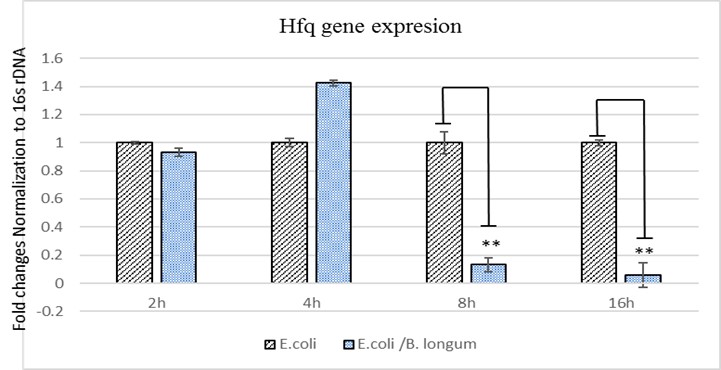
The obtained results from evaluating the expression levels of Hfq showed that B. Longum could inhibit E. coli growth at 8 and 16 hours after co-culturing compared to the cultured E. coli alone (P= 0.0023). However, the observed changes at 2 and 4 hours after co-culturing was not statistically significant (P>0.05). The results were compared using fold-change assessment of expression levels (Figure 4).
The results of the current study demonstrated that probiotic B. Longum not only inhibited the growth of E. coli but also significantly reduced the expression of Hfq virulence gene. Notably, the reduction in E. coli growth is attributed not only to the production of bacteriocin by B. Longum but also to its impact on reducing the expression of the Hfq gene. It has been shown that reduction in the expression of this virulence gene may be attributed to the subsequent reduction in the growth of pathogenic bacteria.
Investigating the resistance and virulence of pathogenic bacteria is essential and the World Health Organization (WHO) has recently emphasized for monitoring E. coli O157:H7 (16-19). Therefore, the development of innovative therapeutic approaches to control this infection is crucial, and the use of probiotics has gained significant attention in the past two decades (20-24).
The use of antibiotics for the treatment and prevention of infections caused by E. coli has not only led to the development of drug resistance but also has disrupted the natural balance of the beneficial normal flora in the digestive system, making the body susceptible to the various intestinal diseases (25-28). In contrast, todays, the use of probiotic bacteria, which are symbiotic and beneficial microorganisms that combat pathogenic microbes in the living environment, can immunize individuals against disease-causing agents through dietary means (29-31).
Probiotics are live microorganisms consumed as dietary supplements or with fermented dairy products. Their consumption plays a significant role in various parts of the body, including mouth, digestive system, urinary and reproductive system, and upper respiratory system for preventing infections (32-34).
Probiotics include various strains of Lactobacillus, Saccharomyces, Bifidobacterium, Enterococcus, and Clostridium that can be used for both the treatment and prevention of infectious diseases. These probiotics perform through various mechanisms such as antimicrobial substances, organic acids, activation of the body immune system, complement system, and competition for the nutrients with microbial pathogens. They inhibit the growth and proliferation of pathogenic agents in that specific area of the body (35-37).
Numerous studies have investigated the impact of probiotics such as Bifidobacterium angulatum, Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactobacillus casei on the growth rate of E. coli under simultaneous growth conditions. A systematic review by Torabian et al (35) demonstrated that probiotics could inhibit bacterial virulence factors.
Our study uniquely identifies the reduction in Hfq gene expression as a key mechanism through which Bifidobacterium impacts E. coli O157:H7, a mechanism not extensively explored in previous research. Antibiotics have also been employed for this purpose, and several notable studies have been conducted in this regard (9, 16).
What makes the current research unique is examination of the mechanism behind reducing the virulence of the pathogenic bacterium E. coli, particularly the O157:H7 strain. The Hfq gene is influential in the replication of the phage in E. coli. It is noteworthy to mention that although this gene plays a role in the replication of the pathogenic bacterium, it contributes to its virulence as well. The results of this research confirm these reports, as our findings indicate that Bifidobacterium not only inhibited the expression of the Hfq gene but also significantly reduced the growth rate of E. coli in adjacent cultures at different time points. The Hfq protein is a key RNA-binding protein that plays a crucial role in regulating the stress response and virulence gene expression in bacteria. The mechanism of action of the Hfq protein is believed to prevent the silencing of the virulence genes by binding to the certain microorganisms, leading to increased expression of virulence genes such as STX1 and STX2 (4). The use of probiotic bacteria can play a protective role in this context (38-41).
This study demonstrated that B. Longum has a significant inhibitory effect on the growth and virulence of E. coli, particularly by affecting the expression of the Hfq gene. The experiments showed that co-culture of E. coli with B. Longum led to a marked decrease in E. coli proliferation, as evidenced by a two-fold reduction in bacterial growth under aerobic conditions at 37°C. This growth suppression was associated with the presence of bacteriocins produced by B. Longum, which are known to have antimicrobial properties. Further molecular analysis revealed a significant reduction in the expression levels of the Hfq gene in E. coli when co-cultured with B. Longum compared to the control. The downregulation of Hfq in E. coli suggests that B. Longum may interfere with the regulatory pathways controlled by this protein, thereby diminishing the pathogenic potential of E. coli.
The findings highlight the potential of B. Longum as a probiotic agent that can not only inhibit the growth of pathogenic bacteria through direct antimicrobial substances but also modulate bacterial gene expression, specifically targeting virulence factors like Hfq. This dual mechanism of physical inhibition of growth and molecular modulation of virulence underscores the therapeutic potential of B. Longum in preventing and managing the infections caused by pathogenic E. coli strains. This could pave the way for the development of novel probiotic therapies aimed at reducing bacterial infections and their associated complications. In the future studies, it is suggested to investigate the exact molecular interactions between B. Longum and Hfq at the genomic and proteomic levels. Moreover, exploring the potential of B. Longum as a probiotic therapy is important to mitigate infections caused by E. coli O157:H7. It is better to assess the impact of B. Longum on other virulence factors regulated by Hfq to provide a comprehensive understanding of its therapeutic potential.
The authors would like to present their gratitude to the Tabriz University of Medical Sciences, Tabriz, Iran for supporting this study.
Ethical Considerations
None.
Authors’ Contributions
Sobhan Aboulhassanzadeh, Samin Aboulhassanzadeh, Zahra Nikkhooy: data collection and analysis. Sobhan Aboulhassanzadeh, Hamed Aghazadeh, and Behboud Jafari: writing manuscript.
Conflicts of Interest
There is no grant support or financial relationship associated with this study.
Not applicable.
All authors reviewed and approved the final version of the manuscript.
All relevant data can be found within the manuscript.
The online version contains supplementary material.
Received: 2024/08/11 | Accepted: 2024/11/20 | ePublished: 2024/11/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |