BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2389-en.html


 , Maryam Yazdanizad2
, Maryam Yazdanizad2 

 , Seyed Davood Mousavi Nasab3
, Seyed Davood Mousavi Nasab3 
 , Delaram Doroud4
, Delaram Doroud4 

 , Rahman Shokri1
, Rahman Shokri1 

 , Ramin Mazaheri Nezhad Fard5
, Ramin Mazaheri Nezhad Fard5 


2- Department of Medical Biotechnology, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
3- Department of Arboviruses and Viral Hemorrhagic Fevers, Pasteur Institute of Iran, Tehran, Iran
4- Deputy of Research, Pasteur Institute of Iran, Tehran, Iran
5- Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ,
In recent decades, prevalence of seafood-associated infections has been increased in Iran as consumption of seafood has generally increased (1). Marine environments can cause surface and tissue contaminations in seafood (2). Seafood infections are caused by a variety of bacteria, viruses, and parasites (3). The outbreaks of seafood-associated infections linked to the polluted waters are majorly caused by calicivirus, hepatitis A virus (HAV), and Salmonella enterica serotype Typhi (2, 4, 5).
Human viruses may infect seafood, however, as commercially used species (fish and shellfish) widely diverge from humans, there is no evidence that they can act as vectors. Therefore, viral infections are mostly limited to the roles of seafood in the passive transmission of viruses (6). Literality, the identified sources of seafood contamination include excessive sewage discharges into the harvesting areas, illegal harvesting of sewage-contaminated water, and sewage collected inlands after the heavy rains or floods (2, 7).
Furthermore, seafood may become contaminated during handling, processing, and preparation steps (8). The storage and transportation at inappropriate temperatures, contamination by the handlers and cross-infection through contact with contaminated seafood or seawater may be other infection contributing factors (2, 7, 9).
Technically, most seafood pathogens are killed or deactivated by thoroughly cooking. Unlike meats and poultries that are often fully cooked, seafood is preferably consumed partially cooked or served under conditions that do not kill microorganisms (2). Foodborne pathogens are still major causes of intestinal infections in humans worldwide, resulting in significant health and economic costs (10). Especially, the importance of controlling these infections on military and passenger-ship sets is high. For example, norovirus has been one of the most important public health challenges for the cruise industry over the last decade (11). In addition, this virus is considered as the most important cause of gastrointestinal infections in the US Navy (12). Nowadays, the global consumption of seafood is significantly increasing due to the rapid increase in the world population and the need for more food sources. Relatively, concerns on food security and general health safety have increased.
Since the recent focuses of administrative regulations have been linked to the development of general health quality, the current study was carried out to investigate distribution of the enteric viruses in raw seafood collected from the stores in Tehran. Based on our knowledge, this study has been carried out for the first time in Iran.
Sample Collection
Raw seafood samples (200 fresh and frozen) in two groups of fish and shrimps (100 fish and 100 shrimp) were collected from the seafood retailers in Tehran, Iran. The samples were immediately transferred to the microbiology laboratory of Pasteur Institute of Iran, Karaj, Iran, under the sterile conditions using cold chain for further analysis.
Primer Design for Virus Detection
For the identification of foodborne viruses, polymerase chain reaction (PCR) primers were designed by Sinaclone, Iran (Primer 7 using PERMANOVA+ software) for six viruses of adenovirus, astrovirus, norovirus, rotavirus, hepatitis A virus (HAV), and hepatitis E virus (HEV) (Table 1).
Table 1. Specifications of the PCR primers for this study.
| No. | Primer | Sequence 5’→3’ | Ref. |
| 1 | Adeno-F | GCCACCGABACGTACTTCAGYCTG | (13) |
| 2 | Adeno-R | GGCRGTGCCGGAGTAGGGTTTRAA | (13) |
| 3 | Astro-F | GGACTGCAAAGCAGCTTCGTG | (13) |
| 4 | Astro-R | GTGAGCCACCAGCCATCCCT | (13) |
| 5 | Noro-F | CTGCCCGAATTYGTAAATGA | (14) |
| 6 | Noro-R | CCAACCCARCCATTRTACA | (14) |
| 7 | Noro2-F | CARGARBCNATGTTYAGRTGGATGAG | (14) |
| 8 | Noro2-R | CCRCCNGCATRHCCRTTRTACAT | (14) |
| 9 | Hepatit A-F | CTCTTTGATCTTCCACAAGRGGT | (15) |
| 10 | Hepatit A-R | GCCGCTGTTACCCTATCCAA | (15) |
| 11 | Hepatit E-F | GGTGGTTTCTGGGGTGAC | (15) |
| 12 | Hepatit E-R | TTCATCCAACCAACCCCT | (15) |
| 13 | Rotavirus-F | GTCACATCATACAATTCTAATCTAAG | (13) |
| 14 | Rotavirus-R | CTTTAAAAGAGAGAATTTCCGTCTG | (13) |
The RNA-PLUS solution (Neday e Fan, Iran) was used to extract the samples. Briefly, 5–10 g of tissues was separated from each sample and completely homogenized using the sterile scissors. Then, RNX-PLUS solution was added to a sterile microtube containing the homogenized sample, mixed by vortex and incubated for 5 min at room temperature (RT). Then, chloroform was added, mixed well, and incubated for 5 min at 4°C. The mixture was centrifuged at 16000 g for 15 min at 4°C. The upper aqueous layer was transferred to a fresh tube and mixed gently with an equal volume of isopropanol for 15 min on ice. Then, the mixture was centrifuged at 16000 g for 15 min at 4°C. The supernatant was discarded and the pellet was dissolved in 75% ethanol. After incubation at 6300 g for 8 min at 4°C, the supernatant was discarded and the pellet was dried at RT. The pellet was dissolved in DEPC water and stored at -80°C. The quality of the extracted RNA samples was assessed using NanoDrop (Thermo Fisher Scientific, USA) with the quantity between 129 and 191 ng/µl.
cDNA Synthesis
The extracted RNA samples were subjected to cDNA synthesis using SinaClon kit (cat no. RT5201). Random hexamer primers, 10Mm dNTP mixture, and RNA samples were mixed and reached to a total volume of 10 µl with DEPC-treated water. The mixture was incubated at 70°C for 5 min, set on ice for 2 min, and then gently stirred. The second mixture was prepared by mixing 5× reaction buffer, M-MuLV reverse transcriptase, RNase inhibitor, and a sufficient amount of DEPC-treated water to achieve a total volume of 10 µl. The mixtures were combined and incubation was carried out at 50°C for 50 min. The reaction was processed by incubating the microtubes for 10 min at 70°C. Microtubes were cooled down on ice and stored at -20°C until use.
Polymerase Chain Reaction
Generally, PCR master mix (SinaClon, Iran) was prepared in a 25-µl total volume, including primers, 20 ng of the template DNA, master mix, and sterile distilled water (DW). The amplification was carried out in a thermal cycling machine using initial denaturation of 5 min at 95°C, following by 40 cycles, each cycle consisted of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 90 sec. Final extension was carried out at 72°C for 10 min. The PCR products were electrophoresed on 1% agarose gel in TAE buffer and visualized under UV light. All the PCR amplifications were repeated to enhance the accuracy.
DNA Extraction and PCR Reaction for DNA virus
Viral genome was extracted using MBST DNA extraction kit (kindly provided by the Institute of Molecular Biological Systems Transfer Research Group, Iran) following the manufacturer's instructions. Briefly, 250 μl of lysis buffer were added to 250 μl of the fecal sample and incubated at 80-90°C for 20 min. Sample was cooled on ice and mixed with approximately 0.3 g of glass beads. Sample was vortexed four times for 4-5 min each. Proteinase K (20 μl) was added to the sample, followed by incubation at 55°C for 2-3 h. Sample was centrifuged at 8000 rpm for 5 min and the supernatant was collected. Then, 360 μl of binding buffer were added to the supernatant and mixed well and incubated at 70°C for 110 min. Moreover, 352 μl of ethanol were added to the mixture and mixed well. This was transferred to a spin column, centrifuged at 8000 rpm for 1 min. Then, 500 μl of washing buffer were added to the column and recentrifuged. Ethanol was removed through an additional centrifugation step at maximum speed. Then, 50-100 μl of elution buffer were added to the spin column, incubated at RT for 3 min, and centrifuged at 8000 rpm for 1 min to collect DNA.
Statistical Analysis
The statistical analysis was carried out using SPSS v.29.0.10 (IBM, USA). Data were recorded as significant when the prevalence values were calculated at 0.05 or less (P<0.05).
Of 100 fish samples, pikeperches, narrow-barred Spanish mackerels, salmons, carps, and javelin grunters included 20 samples each. From the fish samples, 48, 24, 32, 35, 56 and 55 samples were generally contaminated with adenovirus, astrovirus, HAV, HEV, rotavirus and norovirus, respectively. Of 100 shrimp samples, 33, 34 and 33 samples belonged to jinga shrimps, green tiger shrimps, and banana shrimps, respectively. From the shrimp samples, 51, 47, 28, 47, 49 and 47 samples were contaminated with adenovirus, astrovirus, HAV, HEV, rotavirus and norovirus, respectively (Table 2).
Table 2. Comparison of the studied viruses’ dispersions in fish and shrimp samples
| Virus | Dispersal in fish | Dispersal in shrimp |
| Adenovirus | 1/496 | 0/849 |
| Astrovirus | 1/429 | 0/797 |
| Norovirus | 1/449 | 0/842 |
| Rotavirus | 1/359 | 0/770 |
| Hepatitis A virus | 1/554 | 0/875 |
| Hepatitis E virus | 1/262 | 0/751 |
Higher numbers indicate more dispersion.
Comparison of Fish and Shrimp Samples
-
Comparison of Fish Samples viruses
Based on the reports, the significant levels of chi-square tests regarding the adenovirus, HAV, and astrovirus were obtained 0.095, 0.061, and 0.397, respectively, which indicated no significant relationship between these viruses and fish samples. However, this value was obtained 0 for the norovirus, HEV, and rotavirus, which showed significant association between these viruses and fish samples.
The highest and the lowest proportions of norovirus positivity were detected in javelin grunter (40%) and salmons and carps (9%), respectively. Regarding the HEV, the highest proportion was reported in pikeperch and the lowest in javelin grunter, which accounted for approximately 37 and 3% of the total positive contamination cases of HEV in fish, respectively. Furthermore, the highest and the lowest proportions of rotavirus positivity in fish were obtained in pikeperch and javelin grunter, including 30 and 10%, respectively of the total positive contamination cases of rotavirus in fish. Data are shown in Tables 3 and 4 and Figure 1.
Table 3. Frequencies of the fish samples regarding adenovirus, astrovirus, HAV, HEV, norovirus, and rotavirus
| Fish samples |
Adenovirus | Astrovirus | Hepatitis A virus | Hepatitis E virus | Norovirus | Rotavirus | ||||||
| P | N | P | N | P | N | P | N | P | N | P | N | |
| Pikeperches | 22.9% (11/48) |
17% (9/52) |
16.7% (4/24) |
21% (16/76) |
34.4% (11/32) |
13% (9/68) |
37.1% (13/35) |
10.7% (7/65) |
16.1% (9/56) |
25% (11/44) |
30.4% (17/56) |
6% (3/44) |
| Salmons | 27.1% (13/48) |
11.5% (6/52) |
20.8% (5/24) |
18.4% (14/76) |
6.3% (2/32) |
25% (17/68) |
14.3% (5/35) |
21.5% (14/65) |
8.9% (5/56) |
31.8% (14/44) |
17.9% (10/56) |
20.4% (9/44) |
| Carps | 10.4% (5/48) |
28.8% (15/52) |
29.2% (7/24) |
17% (13/76) |
21.9% (7/32) |
19% (13/68) |
25.7% (9/35) |
16.9% (11/65) |
8.9% (5/56) |
34% (15/44) |
25% (14/56) |
13.6% (6/44) |
| Mackerels | 18.8% (9/48) |
21% (11/52) |
8.3% (2/24) |
23.6% (18/76) |
18.8% (6/32) |
20.5% (14/68) |
20% (7/35) |
20% (13/65) |
32.1% (18/56) |
4% (2/44) |
13.1% (9/56) |
25% (11/44) |
Javelin grunters |
20.8% (10/48) |
19% (10/52) |
25% (6/24) |
18.4% (14/76) |
18.8% (6/32) |
20.5% (14/68) |
2.9% (1/35) |
29% (19/65) |
33.9% (19/56) |
2% (1/44) |
10.7% (6/56) |
31.8% (14/44) |
*P: positive; N: negative
Table 4. Chi-square test results for the enteric viruses’ variables in fish samples
| Virus | AS |
| Adenovirus | 0.095 |
| Astrovirus | 0.397 |
| Hepatitis A virus | 0.061 |
| Hepatitis E virus | 0.002 |
| Norovirus | < 0.001 |
| Rotavirus | 0.005 |
Asymptotic significance (2-sided)
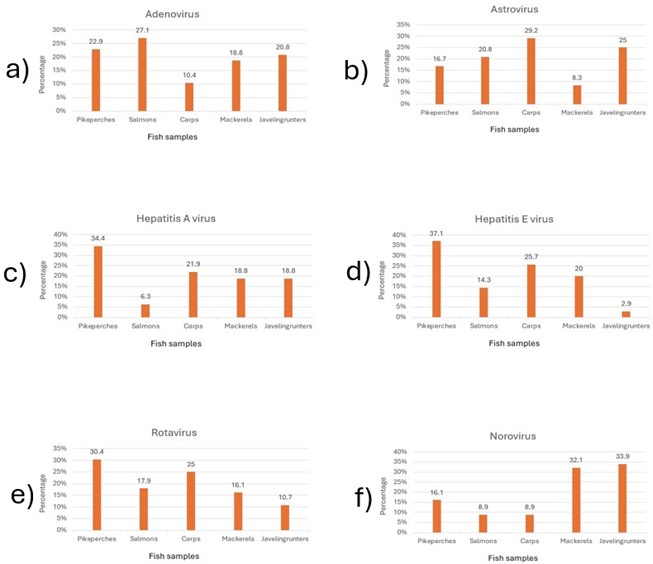
Figure 1. Frequencies of a) adenovirus, b) astrovirus, c) hepatitis A virus, d) hepatitis E virus, e) norovirus and f) rotavirus in the fish samples (Designed by Authors, 2024)
-
Comparison of Shrimp Samples viruses
Based on the reports, the significant level of the chi-square test was obtained 0 for adenovirus, which highlighted the significant association between adenovirus and shrimp samples.
Regarding the other viruses, the significant levels of the chi-square test were calculated 0.258 for rotavirus, 0.073 for norovirus, 0.358 for HAV, 0.1 for HEV, and 0.163 for rotavirus, showing no significant association between these viruses and shrimp samples (Tables 5 and 6 and Figure 2).
Table 5. Frequencies of the shrimp samples regarding adenovirus, astrovirus, HAV, HEV, norovirus, and rotavirus
| Shrimp Samples | Adenovirus | Astrovirus | Hepatitis A virus | Hepatitis E virus | Norovirus | Rotavirus | ||||||
| P | N | P | N | P | N | P | N | P | N | P | N | |
| Jinga shrimps | 47.1% (24/51) |
16.3% (8/49) |
38.3% (18/47) |
26.4% (14/53) |
32.1% (9/28) |
31.9% (23/72) |
29.8% (14/47) |
33.9% (18/53) |
27.7% (13/47) |
35.8% (19/53) |
34.7% (17/49) |
29% (15/51) |
| Green tiger shrimps | 25.5% (13/51) |
42.8% (21/49) |
36.2% (17/47) |
32% (17/53) |
25% (7/28) |
37.5% (27/72) |
44.7% (21/47) |
24.5% (13/53) |
27.7% (13/47) |
39.6% (21/53) |
40.8% (20/49) |
27% (14/51) |
| Banana shrimps | 27.5% (14/51) |
38.7% (19/49) |
25.5% (12/47) |
39.6% (21/53) |
42.9% (12/28) |
29% (21/72) |
25.5% (12/47) |
39.6% (21/53) |
44.7% (21/47) |
22.6% (12/53) |
24.5% (12/49) |
41% (21/51) |
*P: positive; N: negative
Table 6. Chi-square test results for the enteric viruses’ variables in shrimp samples
| Virus | AS |
| Adenovirus | 0.005 |
| Astrovirus | 0.258 |
| Hepatitis A virus | 0.358 |
| Hepatitis E virus | 0.100 |
| Norovirus | 0.073 |
| Rotavirus | 0.163 |
Asymptotic significance (2-sided)
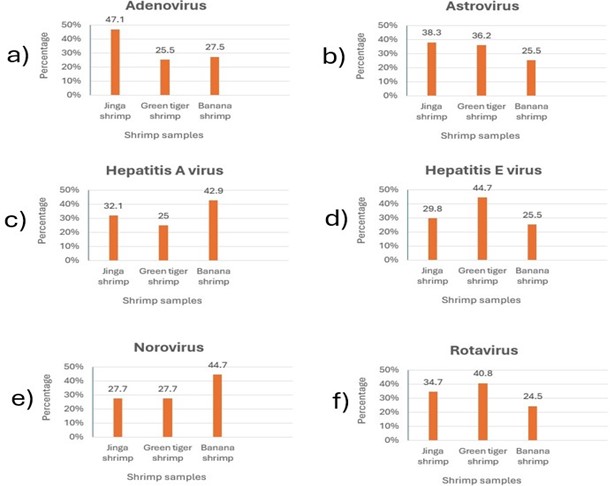
Figure 2. Frequencies of a) adenovirus, b) astrovirus, c) hepatitis A virus, d) hepatitis E virus, e) norovirus and f) rotavirus in the shrimp samples (Designed by Authors, 2024)
Nowadays, several diseases caused by food are still addressed as the most common health problems in the societies. Hence, problems caused by such diseases lead to heavy financial burdens in these societies (16). According to the World Health Organization (WHO), 600 million cases of foodborne diseases are reported annually (17). In most cases, foodborne diseases are caused by inappropriate hygienic measures as well as poor hygienic food preparations (18). In addition, emergence of the new strains of pathogens, changes in the virulence of the known pathogens, novel consumption habits and increase in the population of elderly people and other at-risk age groups have all led to increase in foodborne diseases worldwide (19, 20).
Enteric viruses are important human pathogens that can continuously exist in the environment, including adenovirus, rotavirus, astrovirus, norovirus and hepatovirus (21). Spread of the viral infections is associated with the consumption of contaminated water and food, including oysters contaminated with sewage (22). As viruses cannot be completely removed by the sewage treatment plants, these pathogens spread through water and cause waterborne epidemics (23). Therefore, seafood harvested from the coastal locations are vulnerable to contamination by the enteric viruses, which are of the potential health risks (24). For example, a type of marine edible mollusks of bivalve mollusks can be an effective vector in transmission of the gastrointestinal disease agents to humans; because these mollusks collect their food by filtering small particles from the water. Therefore, these aquatic animals are able to concentrate human pathogens resulting from water pollution. It is well-known that the most common disease associated with the consumption of this shellfish is gastroenteritis caused by norovirus (NoV) (25). Other gastroenteric viruses such as astroviruses have occasionally been observed in this shellfish; although their true epidemiological significance is unclear (24).
In this study, 55% and 47% of the fish and the shrimp samples were contaminated with norovirus, respectively. Norovirus is one of the most common causes of acute diarrhea and vomiting in humans (26), which is majorly transmitted through the orofecal route and often causes diseases through food production or preparation with sewage-contaminated water. This virus is extremely infectious that only a few viral particles are sufficient to cause the infection (27). Norovirus causes relatively mild gastroenteritis with symptoms such as nausea, diarrhea, vomiting, fever and abdominal pain (28). This virus was previously known as Norwalk virus (26). In a study by Yilmaz et al., in Turkey in 2010, the extent of norovirus contamination in shellfish was investigated (29). Matson reported an epidemic in the US marines in 2003 during the US-Iraq War, which was later identified as norovirus disease (30). Riddle investigated the incidence of gastroenteritis in the US naval fleet in the Persian Gulf, 2000–2001, stating that gastroenteritis was a major problem in the naval fleet (31). During a study on the symptoms of a disease in a military base in Germany, Wadl et al., in 2009 reported a complexity caused by the contaminated food and the causative agent was diagnosed as norovirus. Out of 815 people in this study, 101 people became sick within six weeks (32).
In our study, 32% of the fish samples and 28% of the shrimp samples were contaminated with HAV. Generally, HAV is one of the most serious foodborne viruses and the most widely studied virus in food (33, 34). The first detection of HAV in food was carried out in the 1990s and most studies focused on the detection of this virus in seafood named Di Cola (5). It has widely been documented that shellfish is one of the most dangerous food when taken raw or undercooked (33). After analyzing data, Hyun et al., stated that salted clams are sources of HAV contamination. Moreover, they reported that HAV was detected in 8.9% of the shellfish collected in Italy and in 3.8% of clams collected from fish farms and stores in Thailand (35).
In the present study, 35% of the fish and 47% of the shrimp samples were positive for HEV. In general, HEV is one of the causes of viral liver infections. This virus is transmitted through the digestive system via contaminated water and food of animal origins (36). Thus, inadequate treatment of wastewater can contaminate shellfish with HEV. Global studies have shown that HEV is present in bivalve shellfish. Treagus et al., detected HEV in shellfish meat because of water contamination (37). La Rosa et al., reported that 2.6% of 384 shellfish samples of various species from production areas were positive for HEV (38).
In the current study, 48% of the fish and 51% of the shrimp samples were found to be positive for adenovirus. In addition, 24% of fish and 47% of shrimp samples were positive for astrovirus. Ghalyoun et al., investigated the level of shrimps contamination with astrovirus and adenovirus in Istanbul ports. By studying 80 shrimp samples, 85%, 95%, and 80% collected from the local seafood markets were positive for adenovirus, astrovirus, and both viruses, respectively (13). In 2018, Vos and Knox reported the presence of adenovirus in African shellfish (39). In addition, Benabbes et al., identified adenovirus in 52.3% of the Moroccan shellfish samples (40).
The present study found 56% of the fish samples and 49% of the shrimp samples contaminated with rotavirus. Rotavirus is a common cause of diarrhea and diarrhea-related deaths worldwide. It is transmitted primarily via the orofecal route, with symptoms typically developing 1–2 days following infection (41). Marinho et al., identified rotavirus in 26% of total bivalve mollusk samples collected from nine producing fields in the Northern Amazon region of Brazil (42).
Overall, the results from other studies support those obtained in the present study. To control the spread of foodborne diseases in other aquatic communities of the country, the basic measurements are strongly suggested. Further studies are needed to purify disease-causing viruses and carry out genomic sequencing of the viruses to analyze their phylogeny. Necessary plans for the production of the rapid diagnostic tests as well as molecular techniques such as RT-PCR, nested PCR, and real-time PCR should be made. Direct testing of productive fish and removal of contaminated carriers from marine and cultured aquatic populations especially in the breeding centers is very helpful. There was also limitation for this study that was linked to the poor collaboration of the retail-shop owners.
In general, the lack of epidemiological information and limited knowledge about how marine products are contaminated with enteric viruses are effective complications of disease control measurements. Therefore, strict health measurements, including prevention, control and direct testing of the aquatic animals and removal of the contamination carriers should be adopted. Thus, epidemiological and genotypic studies of the foodborne viruses in the farmed and wild fisheries are needed for better understanding of their infection mechanisms and adopt the appropriate prevention strategies.
The study was financially supported by Tehran University of Medical Sciences (grant no. 1400-3-223-56079). The authors thank the staff of the microbiology laboratories.
Ethical Considerations
The current study was approved by the Ethics Committee of Tehran University of Medical Sciences (ethic code no. IR.TUMS.SPH.REC.1400.337).
Authors’ Contributions
Conceptualization: Ramin Mazaheri Nezhad Fard and Golshid Javdani Shahedin; Methodology: Seyed Davood Mosavi Nasab; Statistical analysis: Golshid Javdani Shahedin; Original draft preparation: Delaram Doroud; Review and editing: Rahman Shokri and Maryam Yazdanizad.
This study was supported by a grant from the Food Microbiology Research Center (FMRC), Tehran University of Medical Sciences (grant no. 56079-223-3-1400).
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2024/05/1 | Accepted: 2024/07/27 | ePublished: 2024/08/18
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





