BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2378-en.html
2- College of Dentistry, Al- Farahidi University, Baghdad, Iraq
3- College Engineering, Al- Nahrain University, Baghdad, Iraq
Antibiotic resistance is a challenge we are facing with bacterial pathogens that cause high morbidity and mortality rates. Antibiotic resistant Gram-positive and Gram-negative bacteria have multidrug resistance patterns that make them even resistant to common antibiotics. Due to the lack of efficient prevention measures, the paucity of new antibiotics, and the limited availability of therapies, the situation of today medicine requires the creation of novel treatment alternatives (1). Broad-spectrum antibacterial properties have been demonstrated by nanoparticles so far.
The most frequently used platform in the modern material science study in the 21st century is nanotechnology. The word "nano" stems from a Greek word that means "dwarf." Nanotechnology makes use of formations with dimensions mostly in nanometre scale (1–100 nm). Nanotechnology is becoming a field that is growing quickly because of its applications in science and technology to produce new materials at the nanoscale. One term for nanomaterials in modern medicine is "wonders." Antibiotics are said to be able to eradicate about six different disease-causing organisms, but nanomaterials are said to be able to eradicate about 650 bacteria (2). Also, antibiotic resistance has increased and there is an unmet need to replace them by more applicable and biocompatible compounds (3).
According to earlier reports, metal nanoparticles (NPs) have been the subject of substantial research due to their unique capabilities, including antibacterial wound healing, optical, electrical, magnetic, and catalytic appliances (4). These features have not been found in bulk phase. The various NPs are employed in many applications; Zinc oxide nanoparticles (ZnO NPs), for example, are widely utilized in sunscreen lotions and cosmetics due to their effective UV-A and UV-B absorption capabilities without dispersing visible light. ZnO NPs have also been applied in agriculture, anticancer treatments, and antibacterial experiments (5). ZnO NPs are being produced at a daily rate, which has led to an increase in unintentional human and animal exposure (6). Environmentally acceptable substitutes for chemical and physical processes in the creation of NPs have been proposed such as microbes, enzymes, plants, and/or plant extracts (7).
Pomegranate peels contain significant amounts of phenolic compounds, such as flavonoids (anthocyanins, catechins, and other complex flavonoids) and hydrolyzable tannins (punicalin, pedunculagin, punicalagin, gallic, and ellagic acid). The peels also have an interior network of membranes that makes up approximately 26–30% of the total weight of the fruit. Pomegranate peel (PoP) and juice contain rich forms of these chemicals, which are responsible for 92% of the fruit antioxidant action (8).
Therefore, there is a need for new green synthesis techniques that are straightforward without the need to a lot of energy or hazardous chemicals. Many studies have been conducted recently to look at the bio-reduction of different metal ions such as Ag, Au, Pd, and Pt into metal NPs. In order to convert metal ions into zero-valance metal NPs, the bio-reduction method takes advantage of the reduction potential of a variety of biochemicals found in natural and renewable materials such as microorganisms and plant extracts. There also have been reports of ZnO green synthesis and their antibacterial capacities (9). Studies have employed zinc nanoparticles against bacteria other than the ones employed in this investigation, such as S. aureus, E. coli, K. pneumoniae, B. cereus, E. faecalis, E. faecium, and S. pneumonia (10).
NPs are becoming more and more significant in the scientific research because of their peculiar size and properties. They play a crucial role in many applications and create tremendous changes in various technological fields (11). Researchers have become increasingly interested in nano-biotechnology in particular. Numerous metal oxide NPs are being developed by nano-biotechnology for use in tissue engineering, pharmaceuticals, medication delivery, cosmetics, and biomedical applications (12, 13). Many researchers are currently striving to create biocompatible medication that is effective in treating both cancer and a variety of infectious disorders (14, 15). Nano-compounds have significant intrinsic characteristics that set them apart from larger particles (16).
Because of their antibacterial properties, NPs are now regarded as nano antibiotics (17). People use NPs in a variety of industrial, health, food, space, chemical, and cosmetics applications, which calls for a green and environmentally friendly approach to their synthesis (18, 19). The aim of this study was to prepare ZnO NPs from pomegranate peels in an inexpensive and environmentally friendly way. The prepared ZnO NPs were tested against S. mutans. In their reaction with eugenol, they showed the ability to improve the effectiveness of temporary dental fillings regardless of the tooth condition.
2.1. Preparation of plant extract
Pomegranate peels (Punica granatum L.) were obtained from a market in Baghdad, washed well, and submerged in water to get rid of the impurities. After that, the veneer was completely air dried to eliminate any remaining moisture. Next, 5 gr of the peels were weighed, chopped, and placed in an oven at 50⁰C for approximately 1 hr. The dried peels were crushed in a mortar and pestle, and then placed in a beaker by adding 100 ml of deionized water. The resulting mixture was stirred at 60 to 70⁰C for 1 hr before leaving at room temperature overnight. Whatman No. 1 filter paper was used to filter the extract (Figure 1) (20) and stored at 4°C for further use.

Figure 1. Pomegranate peels extract
2.2. Synthesis of ZnO NPs
Zinc acetate solution (100 ml) (0.1 M) was mixed with an equal volume of plant extract solution. The resulting mixture was heated to 80°C for 180 min and the color change was monitored. As seen in Figure 2, the liquid color changed when the plant excretion came into contact with zinc ions, demonstrating that zinc was absorbed at the nanoscale. As seen in Figure 2, the mixture was heated to 60°C in order to produce the powder form.

Figure 2. Synthesis of ZnO NPs
2.3. Nanoparticle characterization
The biosynthesized ZnO NPs were described utilizing UV-visible spectra, FT-IR spectroscopy, and X-ray diffraction (XRD) spectra. Also, the morphology of NPs was examined using TEM.
2.4. Bacterial media and growth conditions
Streptococcus mutans (serotype C) isolated from carious dentine was acquired from Al-farahidi University dental clinics. S. mutans was grown for 20 hr at 37°C in an anaerobic environment using brain-heart infusion (BHI) and deMan, Rogosa, and Sharpe (MRS) medium (BD Difco, Franklin Lakes, NJ). Using BHI agar plates supplemented with 1.75 μg/ml polymyxin B, 0.3 U ml 1 bacitracin, and 0.005% crystal violet, S. mutans was selectively isolated.
2.5. Method of bacterial growth inhibition
The disk diffusion assay was used to detect the biological activity of the prepared NPs. ZnO NPs were used at 100 µg/L concentration (the highest concentration of the prepared nanocomposite) loaded onto Whatman paper at 37⁰C and incubated for 24 hr. The resulting diameter of bacterial inhibition was then recorded.
2.6. Application of ZnO NPs to dental in vitro
The green synthesized ZnO NPs was mixed with eugenol instead of chemically prepared zinc oxide as a temporary dental filling in the same proportions used in usual methods. Then, the tooth (regardless of tooth condition) was treated in the laboratory in the same way as used to treat tooth decay inside the patient’s mouth, as shown in Figure 3.
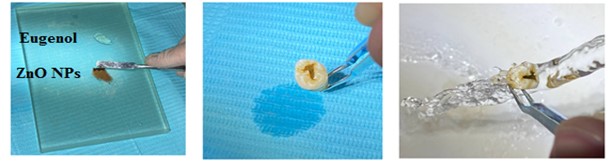
Figure 3. Applications of ZnO NPs to dental in vitro
3.1 Characterizations of ZnO NPs
3.1.1. UV-visible spectrum
ZnO NPs optical absorption characteristics were examined using UV–vis spectroscopy. Employing a Shimadzu UV 3600 UV-vis-NIR spectrometer (Kyoto, Japan: Shimadzu Corporation), the sample UV–vis absorption spectra was captured in the 200–1100 nm wavelength range. The production of ZnO NPs was suggested by the peak at 369.12 nm (Figure 4).

Figure 4. UV-visible spectrum of ZnO NPs
3.1.2. FT-IR spectrum
The FT-IR spectrum of powdered ZnO NPs was obtained in the range of 400-4000 cm-1. The FT-IR of ZnO NPs was shown in Figure 5.

Figure 5. FT-IR spectrum
3.1.3 X-ray diffraction analysis
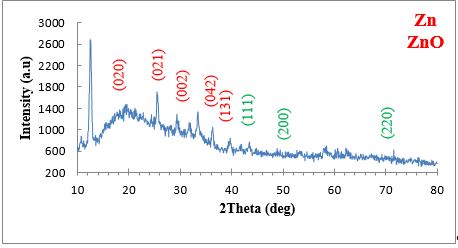
The XRD pattern for the ZnO NPs generated from plant extract was shown in Figure 6. Through XRD examination, the ZnO NPs thickness, phase identity, and crystalline nature were evaluated. The planes (111), (200), and (220) and the peaks at 3 angles (43.8°, 50.9°, and 74.4°) are all caused by zinc. The XRD analysis confirmed production of ZnO NPs. At angles of 18.3, 24.4, 33.6, 35.2, and 42.7, monoclinic crystallite formation is shown by the XRD analysis by the planes (020), (021), (002), (042), and (131), as shown in Table 1.
Figure 6. XRD spectrum of ZnO NPs
Table 1. X-ray diffraction results of ZnO NPs
| Sample | 2θ (Deg.) | hkl | FWHM (Deg.) | K. |
Cos |
C.S | Avg. | |
| Zn | 43.84 | (111) | 0.5900 | 0.1380 | 0.017 | 0.928140 | 14.53623 | 11.011263 |
| 50.94 | (200) | 1.1801 | 0.1380 | 0.017 | 0.903390 | 7.467250 | ||
| 74.44 | (220) | 0.9055 | 0.1386 | 0.0174 | 0.797510 | 11.03025 | ||
| ZnO | 18.3 | (020) | 1.1808 | 0.1386 | 0.0174 | 0.987350 | 6.832274 | 19.47941 |
| 24.4 | (021) | 1.1808 | 0.1386 | 0.0174 | 0.977550 | 6.900766 | ||
| 33.6 | (002) | 0.1968 | 0.1386 | 0.0174 | 0.957570 | 42.2684 | ||
| 35.2 | (042) | 0.3444 | 0.1386 | 0.0174 | 0.953470 | 24.25728 | ||
| 42.7 | (131) | 0.5904 | 0.1386 | 0.0174 | 0.931780 | 14.47941 |
3.1.4 Transmission electron microscopy (TEM) evaluation
As shown in Figure 7, transmission electron microscopy (TEM) was used to examine the shape, size, and morphology of the ZnO NPs.

Figure 7. TEM images of ZnO NPs (a) 30 nm, (b) 60 nm.
3.1.5 Antimicrobial assay
Disk diffusion assay was performed for the evaluation of antimicrobial activity of ZnO NPs against Gram-positive S. mutans. The bacteria were affected by ZnO tiny particles at an intensity of 100% and the percentage of inhibition for S. mutans was 29 mm (Figure 8).

Figure 8. Inhibitory effect of Zno NPs in 100% concentration for S. mutans.
In this study, zinc oxide nanoparticles were produced using Punica granatum L. extract and its antibacterial effect was assessed against S. mutans.
The first indication of plant-based biogenic synthesis of ZnO NPs using P. granatum peel aqueous extract is the color shift from dark red to yellowish white. Following a whole day, the highest color change was achieved. It was caused by the activation of plasmon vibrations on the metal and metal oxide-NP surfaces.
UV-Vis spectroscopy was used to identify the maximum absorbance, which is a measure of the maximum SPR unique to ZnO NPs. The collective oscillation of resonant electrons in the conduction band along the electromagnetic field gave rise to SPR peaks in the UV (21). Here, it was discovered that the phyto-fabricated ZnO NP maximum SPR was 330 nm (Figure 4). The range of light absorption of ZnO NPs at a wavelength of 350–380 nm is associated with the found absorption peak. The strongest SPR band of ZnO NPs produced by P. granatum floral extract was recorded at 345 nm in a recent study (22). The ZnO NPs made from the aqueous extract of P. granatum leaves, on the other hand, showed two peaks at 284 and 357 nm (23).
Figure 5 illustrates how the final material, ZnO NP, was created using peel aqueous extract and examined using FTIR. The O-H vibration peaks overlapped with the NH vibrations, appearing as a broad band at 3549.02 cm−1 (24). Zn was produced with various internal small bands at 3379.29 and 3232.70 cm−1. This attests to the synthesis of primary and secondary amines in addition to OH groups (25). The Zn control material showed the C-H aliphatic at 2931.80. For ZnO material, thiol stretching bands, which were absent from the control sample, are associated with a peak at 2665.62 cm−1 (26). The ZnO NPs have a peak at 2125.56 cm−1, which is associated with the medium sulfonic group stretching bands. The ester group is connected to the ZnO NPs peaks at 1747.51 cm−1. Additional notable peaks for the ZnO NPs sample were observed at 1437 cm−1 for the organic sulfate groups and carboxylate ions, while the peak at 1539.20 cm−1 is associated with the stretching C–C and the low-intensity NH band of primary amines, indicating that binding with ZnO ions occurred (27).
The plant aqueous extract has peaks at 1022.27 cm−1, which are associated with asymmetric C-O-C and C-N stretching. The formation of peaks in the 450–700 cm−1 range, which are absent in the plant aqueous extract, confirms the stretching band of zinc and oxygen and shows that ZnO was successfully formed (28). The function of different functional groups associated with distinct metabolites in ZnO NPs reduction, capping, and stability was demonstrated by their presence in plant aqueous extract.
The findings of X-ray diffraction on the powder form of phyto-synthesised ZnO NPs in the two theta value ranges of 10–80° are shown in Figure 6. XRD can show the crystalline structure of produced NPs, in accordance with the Joint Committee on Powder Diffraction standard (JCPDS). ZnO NPs were found to exhibit prominent diffraction peaks at two theta values of 18.3°, 24.4°, 33.6°, 35.2°, and 42.7°. These values were correlated with crystal planes of (020), (021), (002), (042), and (131), in that order. XRD chart showed crystalline phyto-synthesized ZnO NPs, as opposed to JCPDS card number 36–1451 (29). The acquired observations were compatible with previous studies that show the crystalline character of ZnO NPs generated by plant extract, based on XRD diffraction peaks at the same two theta values (30). The absence of extra peaks on the XRD chart confirmed the high purity of the plant-based ZnO NPs. As previously reported (22), the broad bases of Bragg XRD peaks show that the sizes of synthesized NPs are small. Using the Debye-Scherrer equation, the crystallite size of ZnO NPs was determined in the current study. Utilizing the maximum diffraction peak of (131), which began at a 2° value of 42.7°, the measurement was accomplished.
Researchers frequently utilize the TEM to look at the morphology, size, shape, and agglomeration of the NPs. In the current investigation, zinc acetate was efficiently reduced utilizing the peel aqueous extract of P. granatum to generate spherical ZnO NPs with minimum aggregation (Figure 7). Furthermore, the average particle size of the ZnO NPs were obtained 30.0 nm by TEM images. The particle size from TEM examination and the crystallite size calculated by XRD using the Debye-Scherrer equation were consistent. The findings supported a previous study result that found ZnO NPs made from P. granatum leaf aqueous extract obtained by TEM to be similar in size to those obtained by XRD analysis (31). On the other hand, the TEM analysis revealed the particle size of spherical ZnO NPs, made from an aqueous extract of Citrus reticulata peel, ranged from 23 to 90 nm, while the XRD revealed the crystallite size at 8.9 nm (32).
The antibacterial efficacy of ZnO NPs against the studied bacteria was assessed using a disk diffusion assay at 100% concentration; the percentage of inhibition for Gram-positive bacteria S. mutans was 29 mm.
This study confirmed zinc nanoparticles production from pomegranate peels in an inexpensive and environmentally friendly way. ZnO NPs showed optimal and favorable antibacterial activity against S. mutans. Also, when the ZnO NPs reacted with eugenol, they were shown to have the ability to improve the effectiveness of temporary dental fillings. Green ZnO NPs have emerged as a promising alternative due to their properties against the S. mutans commonly responsible for the tooth decay.
We would like to thank our colleagues who contributed to completing this research.
Ethical Considerations
This study was approved by the Ethics Committee of the College of Dentistry, University of Mustansiriya (Ethical code. 23, 03.06.2023).
Authors’ Contributions
S.M.H. prepared the plant extract. R.H.S. prepared the nano composite. L.H.J. used the nanocomposite in medical applications.
This study was not supported financially by any organization.
Conflicts of Interest
The authors declare no conflicts of interest.
Received: 2024/03/13 | Accepted: 2024/05/20 | ePublished: 2024/05/25
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |