BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2326-en.html

 , Zahra Sabeti Noghabi2
, Zahra Sabeti Noghabi2 

 , Atoosa Haghighizadeh2
, Atoosa Haghighizadeh2 

 , Leila Etemad3
, Leila Etemad3 

 , Vahid Soheili2
, Vahid Soheili2 

 , Omid Rajabi4
, Omid Rajabi4 


2- Department of Pharmaceutical Control, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
3- Pharmaceutical Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran
4- Department of Pharmaceutical Control, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran & Targeted Drug Delivery Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran ,
Biofilm is a bacterial community formed when a group of microorganisms adhere to a surface and shelter inside a polymeric matrix, which is nutritious for the bacterial growth (1). This mini-ecosystem provides a safe environment for the microorganisms, protects them from antibiotics, as well as hosts defense mechanisms and immune responses (2). The arrangement of biofilm matrices varies depending on the microbial species and the growth conditions. Extracellular polymeric substances (EPS), extracellular DNA (eDNA), and proteins generally comprise the biofilm matrix (3). These matrix components are assembled into the supramolecular structures that protect bacteria from unfavorable environmental factors.
The process of biofilm formation is complex, but researchers agree that it can be broken down into a few basic steps: (Step.1) initial contact with and attachment to the surface, followed by the (Step.2) formation of micro-colonies, (Step.3) maturation and development of the biofilm architecture, and finally (Step.4) detachment and dispersion of the biofilm (4).
In spite of the fact that biofilms widely exist in nature, their importance in clinical settings, particularly in light of their role in medical-related infections, is sometimes overlooked (5). In fact, one of the most significant sources of the nosocomial infections is the spread of biofilm of bacterial populations (6). The National Institute of Health (NIH) has indicated that 65% of microbial and 80% of chronic infections are related to the biofilm formation (7).
Due to P. aeruginosa flexibility and strong intrinsic drug resistance, common antimicrobial treatments such as antibiotics, including carbapenems, aminoglycosides, cephalosporins, and ureidopenicillins demonstrate low efficiency against this bacterium (8).
In other words, these bacteria are resistant to the majority of the current antimicrobial treatments due to the bacteria innate resistance mechanisms, such as efflux pumps and multidrug resistance, latent persister cells, and low antibiotic penetration (9). The ability of P. aeruginosa to create biofilms, which protect them from environmental stressors, gives them the capability for colonization and long-term survival. P. aeruginosa has been known as one of the main pathogens in device-associated nosocomial infections (10). Biofilm produced from this pathogen is responsible for 10-11% of all nosocomial infections, and contributes to developing infections in the urinary, respiratory, and wound settings (11, 12). It can also lead to bacteremia, especially in hospitalized patients in the ICU who frequently have hemodynamic instability, and respiratory insufficiency that need mechanical lung ventilation (13, 14). Moreover, combating P. aeruginosa biofilms is critical, especially in hospital settings, because of some other important reasons such as contamination of medical devices, evasion of the host's immune system, increased mortality and morbidity, and the hospital-acquired infections (15, 16).
As of today, ozone therapy has been considered an alternative and non-invasive treatment, which has a number of applications in various fields including medicine, dentistry, water treatment, and food industry (17, 18). The ozonated olive oil is prepared using pure olive oil through ozonation mechanism, in which ozone reacts with the double bonds of carbon in unsaturated fatty acids, and produces different oxygenated species including hydro-peroxides, ozonizes, aldehydes, and di/poly-peroxides, which are responsible for the bacteriostatic and bactericidal activities of the ozonated olive oil (19). This mechanism, ozonolysis, proposed by Criegee in 1975 has been widely accepted by many researchers (20).
Although there are several studies regarding the efficacy of different ozonated oils and water on biofilm elimination, the anti-biofilm effect of ozonated olive oil on P. aeruginosa, which causes device-associated nosocomial infections has not yet been studied. This study aimed to investigate the effectiveness of different concentrations of the ozonated olive oil on P. aeruginosa biofilms.
2-1- Ozonated Olive Oil Preparation
The ozonated olive oil was prepared using pure olive oil through ozonation mechanism, which was discussed in our previous study (21). The physicochemical properties of the extra virgin and ozonated olive oil including iodin index, peroxide value, and acid value were determined by the specific procedures (22). Moreover, to prepare different concentrations of the ozonated olive oil (% v/v), a suitable amount of 100% supersaturated ozonated olive oil was added to the virgin olive oil, to prepare 5%, 10%, 15% and 30% ozonated olive oils.
2-2- Bacterial Strains
Two biofilm-producer strains of P. aeruginosa; POA1 and 1707, were investigated in this study. Both of these strains were obtained from Mashhad University of Medical Sciences, School of Pharmacy. The model strain P. aeruginosa PAO1 is normally used in research of virulence and biofilm stability because of its strong biofilm formation and great resistance to antibiotics and biocides (23). The Persian Type Culture Collection's PTCC 1707 is used to evaluate the antimicrobial therapies since it is a robust biofilm-former and responds well to the new therapeutic approaches (24).
The ability of different concentrations of prepared ozonated oils in preventing the bacteria from biofilm formation, elimination of the constructed biofilm, and finally penetration the biofilm to kill sheltered cells was investigated in this study.
2-3- Biofilm Preparation
P. aeruginosa was cultured on Mueller Hinton Agar (MHA) at 37°C overnight. After that, the bacterial suspension was prepared in sterile normal saline 0.9% and adjusted to 0.5 McFarland turbidity to achieve 108 CFU/mL, and then serially diluted to 106 CFU/mL. For biofilm formation, 180 μL Mueller Hinton Broth (MHB) nutrient medium enriched with 2.5% glucose was inoculated in a 96-well plate and subsequently, 20 µL of 106 CFU/mL microbial suspensions was added into each well. The microplate was incubated at 37°C for 48 hours. During this time, the medium was refreshed every 12 hours and incubated again. After 48 hours, the mixture of microbial suspension and MHB was aspirated and the wells were washed twice with sterile normal saline (25).
2-4- Inhibition of Biofilm Construction
First, 20 μl of each freshly prepared strain of P. aeruginosa (106 CFU/mL) was inoculated separately into the wells containing glucose-enriched culture medium supplemented with various concentrations of the ozonated olive oil. Each concentration was replicated thrice. The plate was incubated for 48 hour and the contents of each well were replaced with fresh ones every 12 hour. Finally, the formed biofilms were stained by crystal violet (0.03%) and the absorbance of each well was determined at 590 nm using a multi-scan plate reader (Biotek, Synergy H4). The wells with higher absorbance indicated more amounts of formed biofilm.
2-5- Degradation of Constructed Biofilm
Similar to the previous method, 20 μL of each freshly prepared strain of P. aeruginosa (106 CFU/mL) was inoculated into the wells containing glucose-enriched MHB. The medium of each well was replaced every 12 hour during 48 hour. In the last 12 hours, the medium was replaced with 200 µL glucose-enriched MHB containing different concentrations of the ozonated olive oil and incubated at 37°C for 12 hours (each concentration was repeated three times). Again, the analysis of the biofilm formation was performed via 0.03% crystal violet staining and the absorbance was determined by a plate reader at 590 nm.
2-6- Quantitative Analysis of Biofilms by Crystal Violet Assay
In brief, after aspiration of the contents of each well, the wells were washed twice with 200 μL normal saline and drained. Next, 50 μl crystal violet was added and incubated for 10 min. Afterwards, the microplate contents were removed and washed twice with 200 μL normal saline to remove unbound dye. In the next step, 200 μL 96% ethanol was added to each well to extract the bound dye. After 10 min, the contents of each well were transferred into the next well to remove the possible unwanted turbidity. Finally, the absorbance of each well was determined at 590 nm (26).
2-7- Penetration of Ozonated Olive Oil into Biofilm
As in the previous experiment, 20 μl of each freshly prepared strain of P. aeruginosa (106 CFU/mL) was inoculated into the wells containing glucose-enriched MHB. The culture medium in each well was replaced every 12 hour during 48 hour. In the last 12 hours, the medium was replaced with a 200 µL glucose-enriched medium containing various concentrations of the ozonated olive oil and incubated again for 12 hours at 37°C. After this period, the wells were replaced with 180 µL glucose-enriched culture medium plus 20 µL 2, 3, 5-triphenyl tetrazolium chloride (TTC) salt (5 mg/ml) and incubated at 37°C for 5 more hours. Finally, the absorbance of the produced red color (due to TTC reduction to triphenyl formazan), indicating the amount of the live bacteria, was determined by the plate reader at 500 nm (27).
As indicated in Table 1, after ozonation, the peroxide value of the oil increased significantly because of the presence of compounds having oxidizing properties. Moreover, the acid value was observed to increase considerably to approximately 17 mg KOH g-1 due to the degradation of by-products after ozonation. In contrast, the iodine index reduced from 81.8 to 0, as a result of a remarkable reduction in the number of double bonds after ozonation (28).
Table 1. Comparisons of peroxide value, acid value, and iodin index in virgin and ozonated olive oil
| Iodine index (g Iodine per 100g) |
Acid value (AV) (mg KOH g-1) |
Peroxide value (PV) (mmol-equv.Kg-1) |
|
| 81.8±1.28 | 0.28±0.02 | 10±0.12 | Virgin olive oil |
| 0 | 17.3±0.06 | 2439±13.3 | Ozonated olive oil |
In this experiment, all concentrations of the ozonated olive oil had significantly different OD values compared to the positive control group (P<0.0001). Moreover, no significant difference was observed between the virgin olive oil and all the ozonated olive oils values against the 1707 strain, meaning that the inhibitory effect of the ozonated olive oils did not depend on the ozone for this strain.
As is shown in Figure 1, for POA1 strain, the 5% and 10% ozonated olive oils had significantly lower OD values compared to the positive control and the virgin olive oil (P<0.0001), meaning that these concentrations were effective in preventing the POA1 strain from biofilm formation. On the other hand, there was not any significant difference between the OD of 15% and 30% ozonated olive oils and their corresponding concentration of the virgin olive oil against POA1 strain, meaning that the increase in olive oil quantity, did not affect the biofilm inhibitory effect.
Therefore, in this method, only 5% and 10% concentrations of the ozonated olive oil prevented the POA1 strain from biofilm formation. In contrast, 1707 strain was resistant against all concentrations of the ozonated olive oil. A similar study conducted in 2024 found that a mixture of olive oil polyphenols, oleocanthal, and oleacein inhibited biofilm formation, virulence factor production, and motility in clinical P. aeruginosa isolates, including multidrug-resistant (MDR) strains. This natural compound mixture disrupted bacterial adhesion and extracellular matrix production, preventing biofilm development. The findings suggest that olive oil polyphenols could be explored as novel anti-biofilm and anti-virulence agents against persistent P. aeruginosa infections (29).
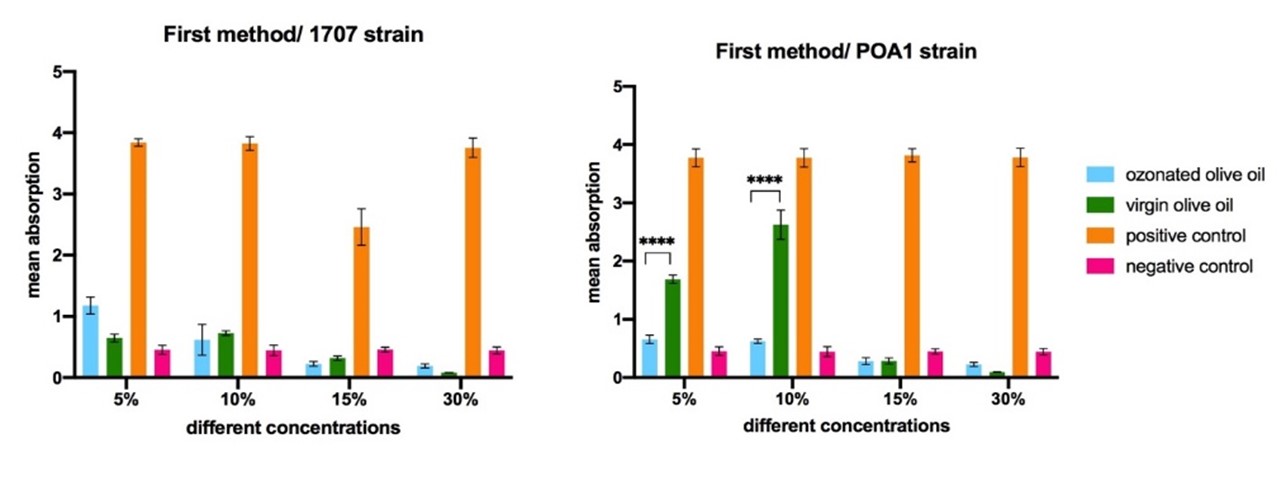
Figure 1. The comparison of the different concentrations of ozonated olive oil in inhibition of biofilm construction. Statistically significant differences are shown as follows: *P<0.05, **P<0.01, ***P<0.001, ****P<0.000.1.
Compared to the positive control, all concentrations of both ozonated and virgin olive oils were significantly effective in the degradation of biofilm for the PAO1 strain. However, the OD value of 15% ozonated olive oil was significantly lower (P<0.0001) than that of virgin olive oil, meaning that this concentration of the ozonated olive oil could successfully remove POA1 biofilm because of the ozone. On the other hand, the similar OD values of the other concentrations of the ozonated olive oils (5%, 10%, and 30%) with the subsequent concentrations of the virgin olive oil confirmed that their ability to eliminate biofilm was not due to the ozone treatment.
For the 1707 strain, the OD value of 5% ozonated olive oil was significantly lower (P<0.0001) than that of the virgin olive oil and positive control, meaning that 5% ozonated olive oil could successfully remove 1707 biofilm. Moreover, there was not any significant difference between the OD values of 10%, 15% and 30% ozonated and virgin olive oil, meaning that the effect of these ozonated olive oils did not depend on the ozone (Figure 2).
In general, while 15% ozonated olive oil eliminated POA1 biofilm effectively, the concentration of 5% successfully removed biofilm of 1707 strain. This is a notable effect as many anti-biofilm compounds are effective only in the pre-construction level of biofilm. For instance, in a study, the ozone-treated vegetable oils were evaluated for their antimicrobial and anti-biofilm effects against methicillin-resistant Staphylococcus aureus (MRSA) from diabetic foot ulcers. The ozonated oils inhibited MRSA strains at concentrations above 4.24 mg/g. They demonstrated moderate to high ability to remove the adhered bacterial cells and eradicate 24-hour-old MRSA biofilms. With their antibacterial and anti-biofilm properties against MRSA, the ozonated vegetable oils showed promise as complementary treatments for the infected diabetic ulcers (30).
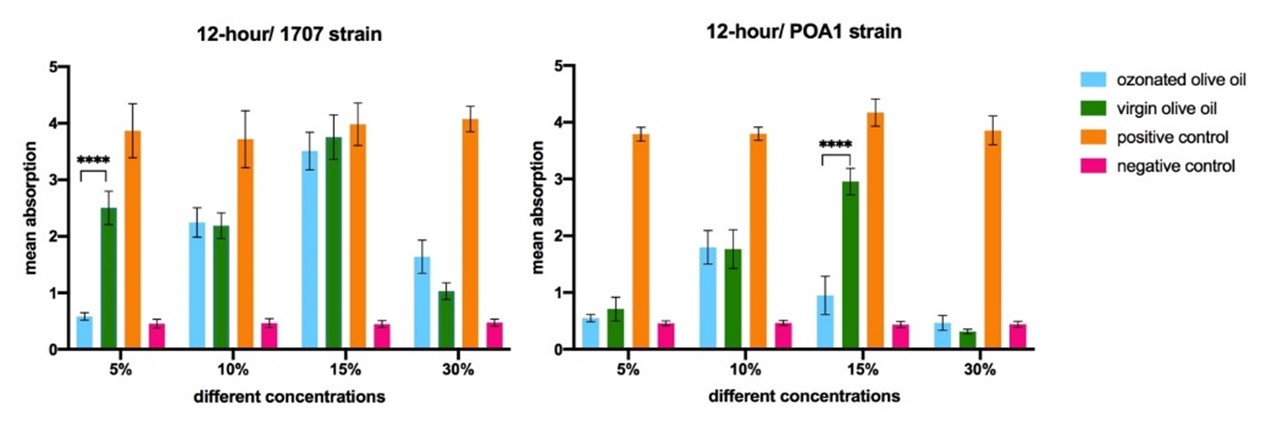
Figure 2. The comparison of the different concentrations of ozonated olive oil in degradation of constructed biofilm. Statistically significant differences are shown as follows: * P<0.05, **P<0.01, ***P<0.001, ****P<0.000.1.
Surprisingly, during this experiment it was found that for the 1707 strain, the virgin olive oil could increase the viable microorganisms of biofilm and the OD values increased compared to the positive control. This effect was almost seen for the other strain, PAO1. However, the OD values of 5% and 15% ozonated oils were significantly lower (P<0.01 and P<0.05, respectively) than that of positive control for the 1707 strain, meaning that these concentrations can diffuse effectively into the biofilm to kill the sheltered microorganisms.
For the POA1 strain, only the OD values of 10%, 15% and 30% ozonated olive oils were statistically lower (P<0.0001, P<0.0001 and P<0.01, respectively) than virgin olive oil and positive control, indicating that they could effectively penetrate the POA1 biofilm.
In general, according to Figure 3, in the penetration assay, all 10%, 15% and 30% prepared ozonated oil penetrated into the POA1 biofilm to kill the biofilm. Additionally, only the 5% and 15% ozonated olive oil could penetrate the 1707 strain biofilm.
Again, this effect is important because the sheltered cells in biofilms are the major causes of recurrent infections. A study showed that biodegradable cross-linked polymeric nanoemulsions (X-NEs) incorporating carvacrol from oregano oil effectively penetrated and killed pathogenic bacterial biofilms. The X-NEs selectively eliminated bacteria without harming the mammalian cells in biofilm-fibroblast co-culture models. Combining natural antimicrobials with the X-NE platform provides a promising therapeutic approach against MDR biofilm infections. The degradable polymers and selective anti-biofilm activity make X-NEs an attractive candidate for the anti-biofilm therapeutics (31).
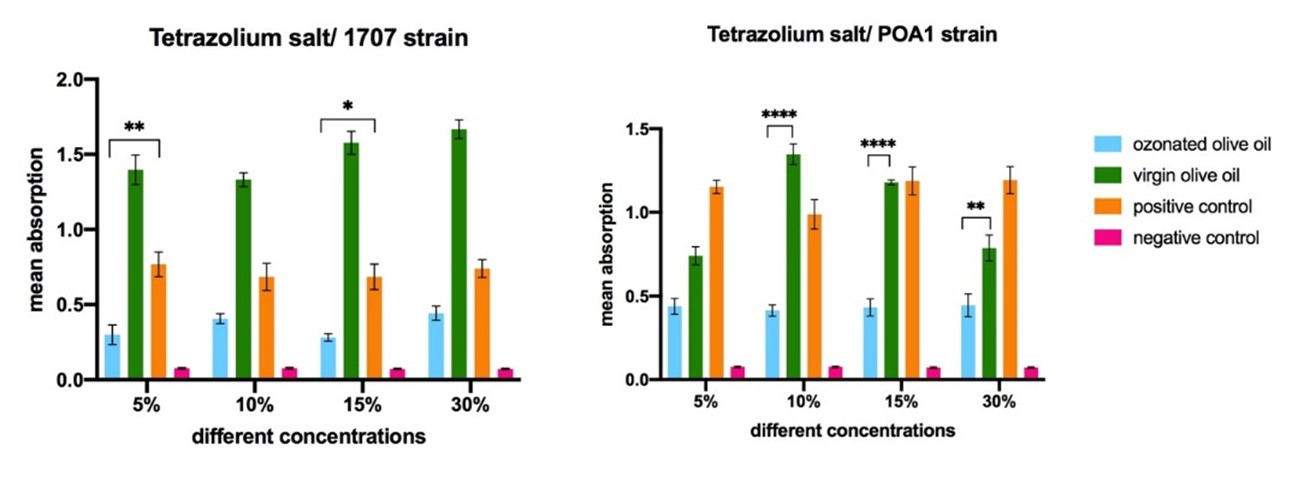
Figure 3. The comparison of the different concentrations of ozonated olive oil in penetration of ozonated olive oil into the biofilm. Statistically significant differences are shown as follows: *P<0.05, **P<0.01, ***P<0.001, ****P<0.000.1.
This study evaluated the anti-biofilm efficacy of the ozonated olive oil against P. aeruginosa biofilms. The key findings were as follow: Ozonated olive oil at 5% and 10% concentrations inhibited biofilm formation by the P. aeruginosa PAO1 strain. The 15% solution completely eliminated pre-formed PAO1 biofilms, demonstrating biofilm degradation ability. While resistant to biofilm inhibition, established biofilms of the 1707 strain were removed by 5% ozonated olive oil. The ozonated oil solutions could penetrate and kill bacterial cells within the PAO1 and 1707 biofilms. In conclusion, ozonated olive oil exhibited promising multi-faceted anti-biofilm effects against P. aeruginosa, including inhibition, degradation, and penetration to the biofilms. This low-cost, eco-friendly product shows potential as an alternative disinfectant for controlling the biofilm-associated infections in the healthcare settings. However, the study focus on only two strains limits the generalizability of the outcome and in vivo evaluations are necessary to assess the real-world efficacy and safety.
The authors thank the Mashhad University of Medical Sciences, School of Pharmacy for the provision of financial support, facilities and equipment for this research.
Ethical Considerations
None.
Authors’ Contributions
Saba Dadpour, Omid Rajabi and Vahid Soheili conceived and designed the experiments. Saba Dadpour, Omid Rajabi and Vahid Soheili wrote the main manuscript text. Zahra Sabeti Noghabi and Atoosa Haghighizadeh performed the experiments. Leila Etemad analyzed the data. Saba Dadpour, Atoosa Haghighizadeh and Zahra Sabeti Noghabi reviewed and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Conflicts of Interest
Received: 2024/06/23 | Accepted: 2024/10/27 | ePublished: 2024/11/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





