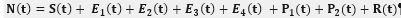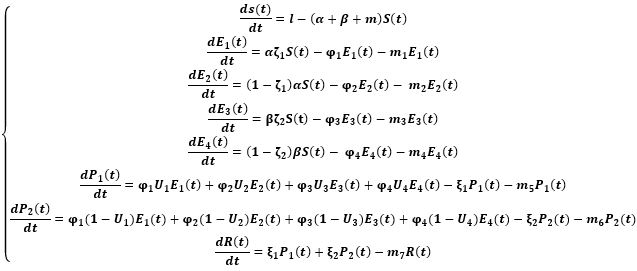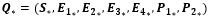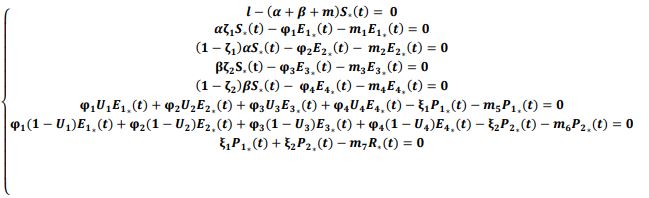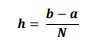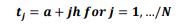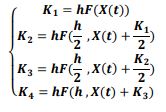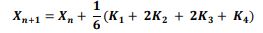BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2320-en.html


 , Shahnaz Rimaz2
, Shahnaz Rimaz2 

 , Maryam Yaghoobi3
, Maryam Yaghoobi3 

 , Sohrab Effati4
, Sohrab Effati4 

 , Mehdi Jabbari Nooghabi5
, Mehdi Jabbari Nooghabi5 

 , Parastoo Tajzadeh6
, Parastoo Tajzadeh6 


2- Radiation Biology Research Center, Department of Epidemiology, School of Public Health, Iran University of Medical Sciences, Tehran, Iran
3- Department of Epidemiology, Faculty of Public Health, Iran University of Medical Sciences, Tehran, Iran & Clinical Research Development Unit, Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran
4- Department of Mathematics, Ferdowsi University of Mashhad, Mashhad, Iran
5- Department of Statistics, Ferdowsi University of Mashhad, Mashhad, Iran
6- Department of Medical Laboratory Sciences, Kashmar School of Medical Sciences, Mashhad University of Medical Sciences, Mashhad, Iran ,
The healthcare-associated infections (HAIs) are serious adverse events predominately emerge in the intensive care units (ICUs) (1). Across different infection types, respiratory events (ventilator-associated events (VAE)) are of particular concern. Defined as pneumonia occurring more than 48-72 hr after endotracheal intubation, VAE happens in about 37, 250 ICU patients in Iran each year (2, 3). Despite the attempts to lower the incidence of VAE, its density still stands at about 6.49 cases per 1000 device days (3). The number of deaths from the HAIs is significant, and the treatment cost for those who recover is extremely high (4). Among microbial agents, Acinetobacter spp. are the most common pathogens causing the HAIs in the ICU patients (5).
Acinetobacter is an opportunistic pathogen found in soil, water, human skin, food products and medical equipment (6). The organism is a non-fermentative, non-mobile, non-fastidious Gram-negative Coccobacillus, capable of growing at various temperatures and pH conditions (7). It utilizes a variety of both carbon and energy sources, making it survive and thrive in hospitals moist or dry conditions (8). Its inherent resistance to an array of antibiotics enables the bacteria to spread in the hospital setting (9). Over the past few decades, the increasing ubiquity and intensity of the mechanical ventilation, central venous and urinary catheterization, and antibiotic therapy have resulted in a burst of Acinetobacter infections in ICUs (10).
The Acinetobacter baumannii (A. baumannii) is a leading cause of severe infections in the ICU patients such as ventilator-associated pneumonia, bacteremia, urinary tract infections, and meningitis. Infections caused by A. baumannii are difficult to treat because of its resistance to different antibiotics (11). Most studies have shown that the most important risk factors in the HAIs are human parameters (medical staff), environmental parameters, and patient-related parameters (6-11).
Nonetheless, the pathways that enable Acinetobacter to transmit in the ICU environment and the potential role of invasive devices are yet to be defined (12).
The acquisition and spread of A. baumanniiis are complex and dynamic process determined by various inter-related factors. Exposure to antibiotics and the resultant intestinal microbiota disruption are known factors to predispose to A. baumannii acquisition. Other major contributing factors for the acquisition of A. baumanniiin in ICUs include patient-related factors such as use of the invasive procedures, and ICU-related factors such as transmission between patients within the ward (cross-transmission) (11). Antibiotics are among the most common drugs prescribed in medicine; nearly 50% of all hospitalized patients and 75% of critically ill patients receive an antibiotic during the hospital stay. However, up to 50% of the prescribed antibiotics are considered inappropriate (13).
Simulation of epidemics has a long history in both mathematics and medical fields. However, calculations vary based on the addressed problems (14). Therefore, artificial intelligence (AI) algorithms with several applications in the field of health care, have been proposed as a solution in many studies in this field, which seems to increase the accuracy of health care data calculations (15, 16).
Using computational and mathematical modelling makes it possible to anticipate the potential disease transmission schemes. Computer simulation and modeling can help overcome several challenges in conducting the ICU research (12).
Automated simulation programs are able to simulate both the short- and long-term effects of the infection control strategies with hundreds or thousands of replications, a valuable feat in the actual hospital environment. The computer model can alter the patient, caregiver, and healthcare setting parameters in a controlled fashion, enabling the investigator to precisely assess the effects of each factor (12). The approach should be able to produce accurate results by modeling the epidemics realistically, which helps to deal with the uncertain dynamics and effects. It can be used for the studies on a specific disease and is suitable for the current research on general issues concerning the analysis of epidemics (14).
The agent-based model (ABM) is one of those models, which simulates the actions, behaviors, and interactions of individuals or agents and measures their impact on the system. The ABM uses the advanced mathematical methods to simulate the dissemination of transmissible agents in the healthcare services (17).
Despite recent advances in computer science, little research has been conducted on the role of the mechanical ventilation in the transmission of nosocomial infections. Identifying the transmission routes will help pinpoint which safety measures are the most necessary to prevent the spread of nosocomial infections. The ABM facilitates the explicit simulation of the healthcare worker– patient interactions that serve as the mechanism for multidrug-resistant (MDR) organisms transmission, and it allows for the distinct representation of the individual characteristics i.e., heterogeneity (11-14).
The intention of this work was to integrate the old and new methodologies in a newly developed framework to provide a flexible, standard, and easy-to-handle approach for modeling a wide class of infectious diseases (14).
Therefore, using an ABM, we attempted to identify the risk factors that could conceivably be implicated in the transmission of Acinetobacter spp. in the ICU settings. To accomplish this goal, an agent-based simulation was designed and developed to model Acinetobacter spp. transmission dynamics and investigate the impact of infection control measures in the ICU settings.
The Iranian Nosocomial Infections Surveillance system (INIS) was utilized as the source of data for modeling. The INIS collects data on the occurrence of the HAIs in the hospital settings using the criteria established by the American Center for Disease Control and Prevention (CDC) and the National Nosocomial Infections Surveillance Guideline (18, 19). Apart from the clinical manifestations and physical examination, microbiological diagnostic tests were undertaken to confirm the diagnosis of the HAIs. The antibiotic therapy was initiated in all patients after determination of the antimicrobial sensitivity of bacteria isolates using the antibiotic susceptibility testing. In this study, the risk factors of the parameters related to the patient that were available were used.
As part of its reporting, it registers information such as subjects' characteristics, the isolated pathogens, and infection sites including ventilator-associated events (VAE), urinary tract infections (UTI), surgical site infections (SSI), skin and soft tissue infections (SST), bloodstream infections (BSI), and pneumonia events (PNE). For the purpose of this study, we reviewed the medical records of the patients who contracted the HAIs during their stay in ICUs of four different hospitals affiliated with Mashhad University of Medical Sciences, Mashhad, Iran, from April 2017 to September 2019. The patients who acquired the HAIs following 48 hr hospitalization in ICUs were included in the study. Those with incomplete hospital records were excluded from the analysis. The collected data comprised age, sex, underlying medical conditions such as cardiac illnesses, digestive system diseases, respiratory diseases, renal complications, neurological disorders and malignancies, causative microorganisms, infection sites, length of stay, and rate of deaths among the patients. Multiple episodes of the HAIs during the hospital stay were counted separately for the data analysis.
2.2 Agent-Based Simulation
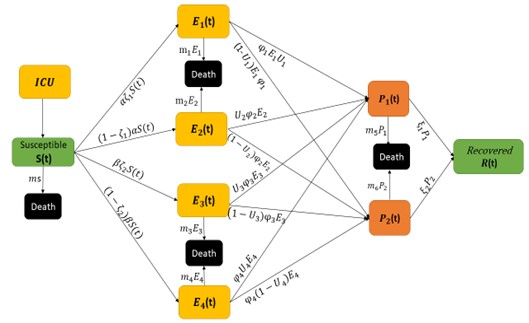
In an attempt to identify the potential role of mechanical ventilation in transmission of Acinetobacter spp. in ICUs, an agent-based mathematical model (ABM) of the patients in a regional network of the hospitals in Mashhad, Iran was designed. The model included differential equations related to the patient-related transmission factors as shown in Tables 1, 2.
Table 1. Patient-related variables used in the mathematics model
Table 2. Parameters used in the mathematical modeling
2.3 Ethical Considerations
The protocol for the current study was reviewed and approved by the Ethics Committee of Mashhad University of Medical Sciences under the code IR.MUMS.REC.1399.331. The anonymity and confidentiality of the patients’ data were taken into consideration during the conduct of the study, in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.4 Statistical Analysis
In this study, after organizing the hospital information in Excel, the R Software was applied. Logistic regression was used for modeling and using other regression models with two-level discrete response, and the method of estimating the parameters of the model of the least error second powers, as well as other methods of error optimization. The relationship between patient-related factors and mathematical simulation methods was performed using Agent-based model, using Net Logo version 5.2. Finally, to evaluate the results, the model was fitted. The fitted model was trained with 80% of the randomly selected data and evaluated for the remaining 20%. If the chance of occurrence predicted by the model is higher than 0.168, the lowest sensitivity is equal to 0.889 and the highest specificity is 0.517. (CI) AUC= 0.747-0.777. Several articles have been validated in this way (11, 13).
A total of 4677 HAI events were recorded in ICUs. The studied population comprised 2395 males (51.2%) and 2282 females (48.8%) with an average age of 55.9±23.36 years. Patients with no underlying diseases (34.6%) cardiac illnesses (16.8%), and respiratory diseases (9.5%) constituted the majority of comorbidities at the time of ICU admission. The Acinetobacter spp. (21.8%) were the most common pathogens isolated from the ICU patients, followed by Klebsiella spp. (13.2%) and Staphylococcus spp. (12.2%). The HAIs primarily occurred in the form of VAE (37.7%), especially among the Acinetobacter spp., where VAE constituted 58.5% of the HAIs. The average duration of hospitalization in the ICU wards was 26.74 ± 15.1 days. The occurrence of the HAIs in 47.7% of the ICU patients eventually led to death (Table 3).
Table 3. Clinical and demographic characteristics of patients with healthcare-associated infections
The number of cases is presented with percentages.
3.2 Design of agent-based model
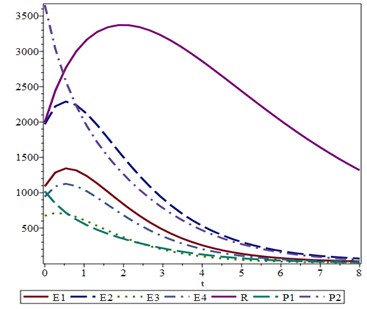
There are a number of factors that can conceivably be implicated in the transmission of Acinetobacter in ICUs: environmental factors, patient-related factors, and physician-related factors (21). For the mathematical modeling, patient-related parameters were implied (Figure 1).
 of the system, the following differential equations were written:
of the system, the following differential equations were written: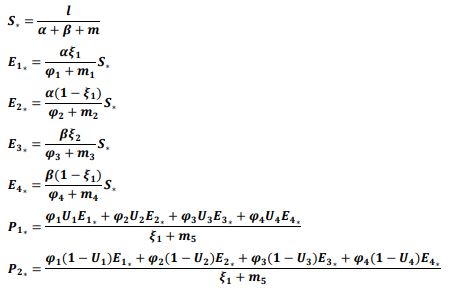
3.3 Numerical Solution

The sensitivity of the model in expressing the changes in the occurrence of Acinetobacter on the model variables and its characteristics showed the area under the rock curve at 0.762 that is almost acceptable.
Nosocomial infections are one of the most common problems in the health care system. Acinetobacter spp. are the most common cause of the hospital infections in the ICUs, which have shown antibiotic resistance, and the most important risk factors are the length of hospital staying, long-term antibiotic use, and underlying diseases that increase the costs and long-term disability and mortality (22-26).
According to the results of studies, patients admitted to the ICUs are 5-7 times more likely to have nosocomial infections (27). There are several factors that could conceivably be implicated in the transmission of Acinetobacter in ICUs that include: environmental factors, patient-related factors, and physician-related factors (11, 23, 28-30), however, the relative importance of each in the healthcare setting is unknown.
For the mathematical modeling of Acinetobacter transmission in the ICUs, in this study, we used ABM to understand the effect of the patient-related parameters. These parameters were calibrated and evaluated using the observed data from 4 independent sites (hospitals) over a period of 18 months. Therefore, data from multiple sites can help to reduce the uncertainty surrounding this calibration process that is available via collection through the randomized clinical trials. We showed that the calibrated transmission probability, the calibrated underlying disease, and invasive devices effect were high compared to the final calibrated parameter values. These results suggest that VAP prevention bundles and guidelines were efficient in reducing the rate of VAP.
The present data indicated that patients with a history of underlying disease and use of invasive devices like mechanical ventilation were more likely to get Acinetobacter infection than other bacteria. The Acinetobacter spp. (21.8%) was the most common organisms responsible for the development of the HAIs in the ICUs of the northeast Iran. The most important way to transfer was through a mechanical ventilation device. Papazian et al (29) demonstrated that ventilator-associated pneumonia (VAP) remains one of the most common infections in the patients requiring invasive mechanical ventilation.
The incidence rates greatly varied based on the studied population. For example, VAP rates were reported as high as 24.5% in the cancer patients but 17.8% in the trauma patients (30-32). The organisms associated with VAP vary according to the several factors including duration of the mechanical ventilation, the length of hospital stays before VAPs, timing and cumulative exposure to antimicrobials. The usual Gram-negative microorganisms involved in VAP were Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia and the major Gram-positive microorganisms were Acinetobacter spp and Staphylococcus aureus (33-42).
In this study, the prevalence of VAP was 37.7%. In a study by Salehifar et al (39), the prevalence of hospital pneumonia was 11.4% (VAP: 91.4% and Non–VAP: 8.6%), and the most common organism was Acinetobacter spp. (22%). In the study of Amri Mele et al (40), the prevalence of VAP was 27.6% from that 24.3% was caused by Acinetobacter spp..
Similarly, in a study by Wang et al (43) in China, in the field of infections related to the invasive devices, ventilator was the most common instrument that caused nosocomial infections.
In a study conducted by Barnes et al (13) using an ABM (with the help of Net Logo software) to investigate the effects of reducing antibiotic use on the transmission of resistant microbes, the results showed that considering the effect of the microbiome, the rate of infection transmission is reduced from 75% to 65% (10%). Considering that about 75% of the patients in the ICUs take antibiotics and 50% of them use it inappropriately, it is expected that the transmission of infectious agents and drug resistance will decrease clinically, even with a moderate decrease in the antibiotic use (13).
In the 2016 study, Doan et al (11) performed an ABM (with the help of Net Logo software). Assuming that 25% of the patients had colonization and 18% had infection, after including the human parameters (hand hygiene) and environmental parameters (environmental cleansing) in the model the results showed that the rate of infections would be reduced by more than 80% (11).
Most studies have shown that the underlying diseases and invasive devices are the most important risk factors for the nosocomial infections in the ICUs (41-44). The results of the studies showed that 90% of the nosocomial infections are caused by bacteria that the type of bacteria is different in various communities. The prevalence and pattern of the HAI-causing microorganisms vary by the hospital, geographic area, and the patient status (44). It is therefore reasonable to expect a slight discrepancy with previous reports on microbiological etiology. Due to the advancement of technology and the occurrence of emerging diseases, it seems that the use of new analytical methods and simulation modeling methods such as ABM that can show the infection tracking will be useful in controlling and preventing the infections.
Besides, preventive measures focus on the modifiable risk factors, mediated by the non-pharmacological and pharmacological evidence-based strategies recommended by the guidelines and bundle. Because of the nosocomial infections potential associated with the coronavirus infection (COVID-19) and increased mortality and costs, the management planning is strongly recommended (45-50).
Seemingly, the use of modeling methods such as agent-based modeling (ABM) along with the disease simulation for intervention and management planning and futurism will be useful and will help to reduce the mortality and costs. In addition, given the conditions in hospitals, it may be possible to design appropriate tools to control the infections. Therefore, due to the high prevalence of Acinetobacter spp. in the study population, considering the guidelines and bundle of the WHO and CDC and the compliance Standard Precautions can reduce the probability of transmitting the nosocomial infections and Acinetobacter spp.
We would like to thank the Vice chancellors for research affairs of Mashhad University of Medical Sciences for the financial support and all those who helped us with this research.
Ethical Considerations
The protocol for the current study was reviewed and approved by the Ethics Committee of Mashhad University of Medical Sciences under the code IR.MUMS.REC.1399.331.
Authors’ Contributions
Babak Eshrati conceptualized, supervised, and administered the study. Shahnaz Rimaz and Maryam Yaghoobi conducted the formal analysis. Parastoo Tajzadeh, Sohrab Effati, Mehdi Jabbari Nooghabi and Maryam Yaghoobi conducted the investigation. Maryam Yaghoobi and Parastoo Tajzadeh wrote the main manuscript text and edited. All authors reviewed and approved the manuscript.
Conflicts of Interest
This work was supported by Mashhad University of Medical Sciences (Grant number 981547).
Received: 2024/05/25 | Accepted: 2024/09/15 | ePublished: 2024/11/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |