BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2312-en.html

 , El-Amin Mohamed Ibrahim2
, El-Amin Mohamed Ibrahim2 
 , Sara Gamal Gubara Mohamed3
, Sara Gamal Gubara Mohamed3 
 , Samah Awad AbduRahim4
, Samah Awad AbduRahim4 
 , Hadeel Gassim Hassan2
, Hadeel Gassim Hassan2 
 , Ali Elbagir Ali Mohamed2
, Ali Elbagir Ali Mohamed2 
 , Leena Babiker Idris Babiker5
, Leena Babiker Idris Babiker5 
 , Hana A Elkhalifa6
, Hana A Elkhalifa6 
 , Marwa M Suliman7
, Marwa M Suliman7 
 , Marwan M Badawi8
, Marwan M Badawi8 
 , Mohamed A. Hassan9
, Mohamed A. Hassan9 
 , Aisha Zoheir Ibrahim Almagboul10
, Aisha Zoheir Ibrahim Almagboul10 

2- Department of Medical Microbiology, University of Khartoum, Khartoum, Sudan
3- Department of Medical laboratory, Mediclinic Airport Road Hospital, Abu Dhabi, United Arab Emirates
4- Department of Microbiology, Faculty of Medicine, Al-Rayan Colleges, Al Madinah al-Munawwarah, Saudi Arabia
5- Department of Psychiatry, Medical Specialization Board, Khartoum, Sudan
6- Department of Medical Hematology, University of Science and Technology, Omdurman, Sudan
7- Department of Radiology, Dallah Hospitals, Riyadh, Saudi Arabia
8- Department of Microbiology, Medical Unit, Higher Academy for Strategic and Security Studies, Khartoum, Sudan
9- Department of Microbiology, Precision Medicine, Sanimed International Lab and Management, Abu Dhabi, United Arab Emirates
10- Department of Microbiology, Medicinal and Aromatic Plants and Traditional Medicine Research Institute, Khartoum, Sudan
Helicobacter pylori (H. pylori) is a spirally rod-shaped micro-aerophilic flagellated Gram-negative bacterium. It was first identified by Marshall and Warren in 1982 associated with gastric disorders. Currently, it is considered as a global pathogen that affects half of the world's population, with higher rate of occurrence in developing countries. It causes various stomach disorders, including gastritis, chronic peptic ulceration, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma, leading to significant morbidity and mortality. Researchers attribute this pathogenicity to the presence of virulence genes such as vacuolating toxin (VacA) and cytotoxin-associated gene Pathogenicity Island (cagPAI) (1-5).
In Sudan; H. pylori was detected in 80% of gastritis samples, 56% among patients with duodenal ulcer and 60% among patients with duodenitis. Moreover, a recent study applied molecular techniques to detect H. pylori from different regions of Sudan, their study indicated that 37.33% of samples obtained from patients experiencing different endoscopic findings are H. pylori positive (6, 7).
Despite decades of effort, eradicating the pathogen remains a challenging task for physicians, as there is no universally effective regimen in addition to the high rate of resistance. The critical problem is that no single drug cures H. pylori infection. The standard triple therapy consists of a proton pump inhibitor, amoxicillin, and clarithromycin. This therapy is used for H. pylori eradication in many countries. However, due to the rising prevalence of antimicrobial resistance, mainly to clarithromycin, many other regimens were developed, including quadruple, sequential and concomitant therapy regimens supplemented with metronidazole, clarithromycin and levofloxacin. Indeed, considerable attempts have been made to resolve such dilemma, yet, resistance to clarithromycin and metronidazole is still critical concern and have led to eradication failure. Furthermore, adding insult to an injury, the side effects of the triple regimens were reported to decrease the treatment compliance as well (8).
Plant extracts especially essential oils (Eos) are used for centuries as antimicrobial agents or food preservatives. Accordingly, several studies have been established to extract various natural products including essential oils and screen their antimicrobial activity (9, 10).
Cymbopogon schoenanthus is one of the most important species of the Poaceae family; its antibacterial activity against both Gram-positive and Gram-negative bacteria has been previously reported (11-13). The Poaceae family also includes Cymbopogon citratus, which exhibits similar broad antimicrobial activity (14, 15). Moreover, Syzygium aromaticum belongs to Myrtaceae family and its bioactive properties and inhibitory effects against wide range of bacteria including H. pylori were reported as well (16-18). Furthermore, Boswellia papyrifera or Sudanese frankincense is a member of Burseraceae family; it has been determined to have high antibacterial as well as antifungal activities (19, 20). Thymus vulgaris belongs to Lamiaceae family and is native to the Southern Europe and the Mediterranean area; it has been verified to have antibacterial and anti-adherent activities against some bacteria (21, 22). Lastly, Cinnamomum verum, which is known as true cinnamon is indigenous to Sri Lanka and Southern parts of India and has been investigated for its antibacterial potential in several studies (23, 24). These herbs not only exhibit bactericidal properties but also demonstrate significant synergistic effects when combined with other herbs or antibiotics. This underscores the potential of such combinations to enhance antimicrobial efficacy, particularly against life-threatening bacteria such as H. pylori (25-28).
Even though combining EOs would minimize the minimum inhibitory concentration (MIC) of their combinations and enhance the activity in synergistic effects, there have been few in-depth studies focusing on their antimicrobial properties (28). The current study therefore initiated to evaluate in vitro antibacterial activity of some EOs combinations against specific H. pylori clinical isolates from patients in Sudan.
Study Design
This prospective cross-sectional hospital-based study was conducted in Soba University Hospital, Khartoum, Sudan from June 2018 to September 2019. All laboratory processes were performed in Microbiology and Molecular Biology labs at Faculty of Medical Laboratory Sciences, University of Khartoum.
Data Collection
Patients with upper gastrointestinal tract (GIT) symptoms especially dyspepsia and heart burn who had referred to Gastroenterology Department for endoscopy during the mentioned period were included after obtaining an informed consent. Patients with a history of using proton pump inhibitors (PPIs), antibiotics, or non-steroidal anti-inflammatory drugs (NSAIDs) were excluded. Patients’ data was first de-identified and then recorded in excel file.
Primary Identification of H. pylori
Duplicated mucosal biopsy specimens were taken from the gastric antrum samples using a disinfected endoscope. Egg yolk emulsion (EYE) agar was used for cultivation with minor modifications (29). Columbia agar base (40.40 gr/L) was also used with 10% egg yolk, 0.25% yeast extract, and campylobacter selective supplement (10 mg vancomycin, 5 mg trimethoprim, 5 mg cefsulodin, and 5 mg amphotericin B per liter). For non-selective cultivation the same media were used without adding selective supplements. Both plates were incubated in a microaerophilic atmosphere composed of 10% CO2 and 5% O2 using CO2 incubator for up to 5 days at 37°C and 100% humidity.
All detected colonies were presumptively identified by morphological features, microscopical examinations, and biochemical tests including urease, oxidase, and catalase tests. The isolated H. pylori colonies were further sub-cultured on blood and chocolate blood agar media supplemented with 0.25% yeast extract for purification purposes.
Molecular Identification of H. pylori
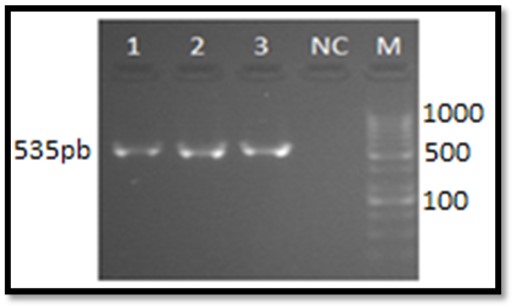
Manual guanidine chloride method was adopted to perform DNA extraction as described previously (30). H. pylori 16S Ribosomal RNA primary tetracycline binding sites gene was used to characterize the isolated strains. PCR was carried out using Maxime PCR PreMix Kit (i-Taq) (iNtRON BIOTECHNOLOGY, Seongnam, Korea). The following primers were used: (F: 5'CTGACGCTGATTGCGCGAA-3ˈ) (R: 5'TGGCTCCACTTCGCAGTATT- 3ˈ) (31). PCR was carried out using thermocycler (SensoQuest, Germany) in 25 µl reaction mixture and the PCR protocol was as follow: 1 cycle at 95°C for 3 min, 30 cycles at 95°C for 1 min, annealing at 58.5°C for 1 min, and 74°C for 1 min, and a final elongation at 74°C for 15 min. The amplified products of 535 bp were detected by electrophoresis on 2% agarose gel in 1x Tris EDTA Buffer (TBE buffer) (89 mM Tris base, 89 mM Boric acid and 2 mM EDTA dissolved in 1 Liter H2O) for 30 min. The gels were stained with 3 µl ethidium bromide (10 mg/ml). A 100MW DNA ladder (iNtRON BIOTECHNOLOGY, Seongnam, Korea) was used in each gel as a molecular size standard. The 16S rRNA gene sequences were submitted in GenBank nucleotide database under following accession numbers: MW599345, MW599346, and MW599347.
Characterization of H. pylori
To determine changes in 16S rRNA, a comparison between conserved regions of primary tetracycline binding sites was conducted; nucleotide positions between 722 and 1234 (numbering is according to the rRNA gene of H. pylori strain 26695) with H. pylori reference strain 26695 and tetracycline resistance H. pylori strain 108 (accession number AY062898.1). Highly similar sequences were retrieved from the NCBI GenBank database and subjected to multiple sequence alignment using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/), Gblocks(http://molevol.cmima.csic.es/castresana/Gblocks_server.html) and BioEdit sequence alignment editor software. The evolutionary history was inferred using the Neighbor-Joining method (32, 33).
Preparation of Bacterial Inoculum for Susceptibility Testing
A BaSO4 turbidity standard equivalent to a 0.5 McFarland standard was used to standardize the bacterial inoculum density for susceptibility test. BaCl2 solution (0.5 ml of 0.048 mol/L) (1.175% w/v BaCl2 • 2H2O) was added to 99.5 ml of 0.18 mol/L (0.36 N) H2SO4 (1% v/v) stock solution with constant agitation to maintain barium sulfate suspension aliquot. The correct density of turbidity standard was verified measuring the absorbance using a spectrophotometer with a 1-cm light path and matched cuvettes. The absorbance for the 0.5 McFarland standard at 625 nm was considered from 0.08 to 0.13 (34). Direct colony suspension method was used for inoculum preparation according to Clinical and Laboratory Standards Institute (CLSI) (35).
Plant Materials Acquisition and Essential Oil Extraction
The plants used in this study were obtained from commercial sources. They were provided from Medicinal and Aromatic Plants and Traditional Medicine Research Institute (MAPTMRI), Khartoum, Sudan. Voucher specimens were affirmed for reference in the herbarium. The botanical names of the tested plants, families and morphological parts used are illustrated in Table 2.
In this study, six essential oils were selected based on their well-documented antimicrobial properties. To ensure a comprehensive analysis, the oils were chosen from various botanical families, each with distinct chemical compositions, allowing for a broader evaluation of their efficacy against H. pylori. The final selection was made following review of existing literature, focusing on oils identified as promising due to their antimicrobial and synergistic effects. The availability and purity of the materials were rigorously assessed to ensure the reliability of the research outcomes. Each plant material was subjected to 4 hr hydro-distillation using Clevenger apparatus to obtain the essential oil. After drying the oil over anhydrous sodium sulfate, filter paper was used to filter it, the oils were then stored in amber vials at 4°C for further use.
Quantitative Evaluation of EOs Antibacterial Activity In Vitro
The MIC values of EOs, both individually and in combinations, were obtained using agar dilution method following CLSI instructions with slight modification (35). Serial two-fold dilutions from the stock of each EO in 100% absolute methanol as a solvent were added to a molten and equilibrated to 45 to 50°C Mueller-Hinton agar supplemented with 5% human blood and 0.25% yeast extract, then mixed thoroughly and dispensed into 10 ml Petri dishes on a level surface to result in an agar depth of 3 to 4 mm. The agar plates were left to solidify at room temperature. Immediately, using pipettes with sterile tips, 5 μl aliquots of bacterial suspensions containing approximately 105 CFU/ml per spot was used to inoculate the surface of the dried agar. Additionally, a drug-free plate was used as control for growth viability and purity. An agar plate containing the same concentration of methanol used for diluting oils, but without oils, was also included to ensure the bacteria viability at methanol concentration.
Determination of EOs Synergism Against H. pylori
In vitro assessment of antibacterial effects of EOs combinations were attempted applying the checkerboard technique. Concisely, the MIC of each EO alone and in combination was determined. Then, the fractional inhibitory concentration (FIC) and FIC index were calculated by summing the separate FICs of single oil solution using following equation:
FIC (A) = MIC (A) in combination/MIC (A) alone.
FIC (B) = MIC (B) in combination/MIC (B) alone.
FIC index = FIC (A) + FIC (B).
Where, FIC (A), FIC (B), MIC (A) and MIC (B) are the FICs and MICs for EOs A and B, respectively. Synergistic activities are defined in a range of FIC index less than 0.75, while antagonism in a range of an FIC index greater than 2; the combination is defined as additive in a range of ≥0.76 (36-38).
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 21.0 statistical software. One-way Analysis of Variance (ANOVA) was conducted, with a 95% confidence interval. Statistical significance was set at p-value of less than 0.05.
In the current study, 70 mucosal antral biopsy specimens were included. H. pylori isolates were detected in 30 (42.9%) rapid urease test. From the positive Campilobacter Like Organism (CLO) test samples; 17 (56.7%) strains were isolated using culture method. All cultured strains gave positive reactions to the routine biochemical tests with typical colonial morphology and Gram staining. Moreover, they were all 16S RNA positive. The 16S rRNA sequenced strains isolated from patients with different pathological features confirmed identification of H. pylori. The genetically identified H. pylori strains were used to evaluate the antimicrobial activity and interactions of EOs as described in Table 1.
Molecular Identification and Characterization of H. pylori
Molecular identification of H. pylori was carried out for three clinical isolates. PCR results showed all of them as 16s rRNA-positive (Figure 1). All genetic fragments were sequenced. The accession numbers obtained from Genbank for the sequences are illustrated in Table 1.
Table 1. The accession numbers of the 16S rRNA clinical isolates of H. pylori
| Isolate No | Accession no. of 16srRNA | Clinical region |
| 23 | MW599345 | Gastric ulcersample |
| 39 | MW599346 | Gastritis sample |
| 41 | MW599347 | Dyspepticun-ulcer sample |
Further analysis of three 16S rRNA sequences isolated from Sudanese patients with different clinical features confirmed identification of H. pylori strains. Blasting these sequences revealed homology of approximately 99%. These sequences were further analyzed for mutations and conservations to determine changes in 16S rRNA. The multiple sequence alignment (MSA) of the conserved region of primary tetracycline binding sites revealed that 515 bp PCR fragment from the three H. pylori clinical isolates from Sudanese cases lacked AGA926-928⟶TTC substitution (Figure 2).
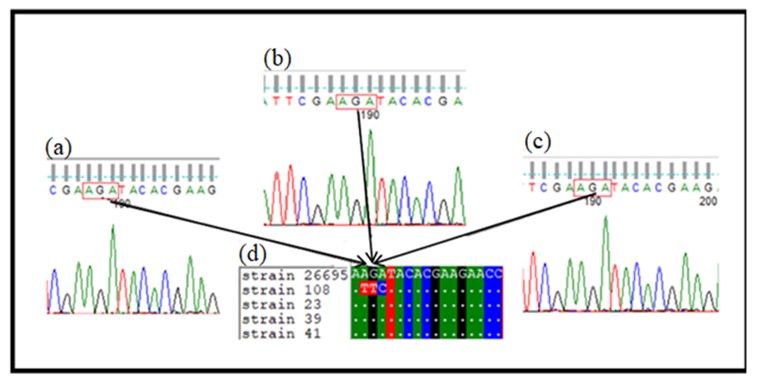
Figure 2. (a), (b), and (c) Show the sequence chromatogram of the aligned strains. The different nucleotides are shown in colored letters in boxes, and white dots indicate similarity. (d) Shows alignment of clinical isolates sequences with H. pylori reference strain 26695 and tetracycline resistance H. pylori strain 108 (accession number AY062898.1)
The alignment of isolated sequences exhibited various sequences that differed in one base-pair substitution in two positions (T1103C and C1128T) (Figure [3c]). The MSA of isolates with H. pylori Genbank strains confirmed the occurrence of variations between the Sudanese isolates and selected published nucleotide sequences (Figure [3b]). The unrooted phylogenetic tree offers significant insights into evolutionary relationships among various strains, including local isolates such as Strain 23-Sudan, Strain 39-Sudan, and Strain 41-Sudan. Divergence of phylogenetic tree resulted in two lineages, as illustrated in Figure 4. Lineage 1 shows clustering of Sudanese strains with those from Mexico and Australia that suggests shared evolutionary pressures. In lineage 2, 16S rRNA sequence of Strain 41-Sudan shared a common ancestor with strains from Venezuela. The branch lengths in the phylogenetic tree indicate genetic divergence, with Strain 41-Sudan exhibiting a longer branch.
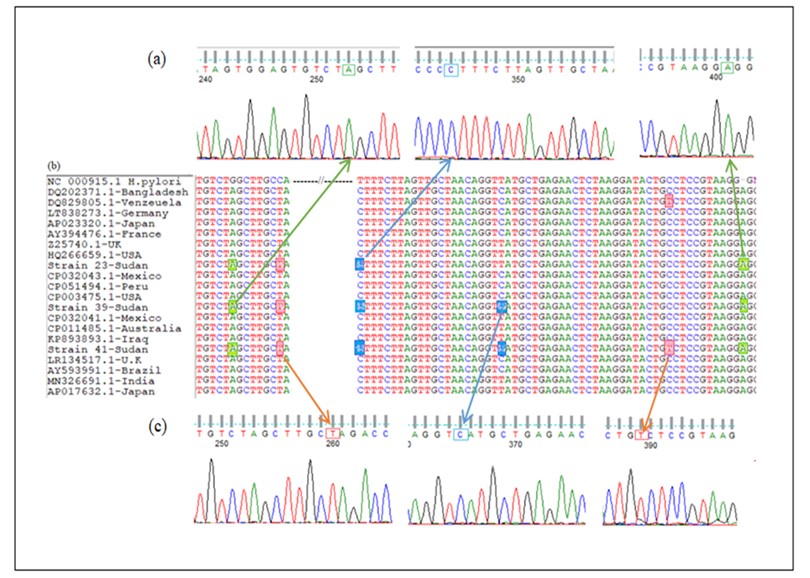
Figure 3. Multiple sequence alignment of 16S rRNA gene sequences with other selected global strains obtained from GenBank. (a) and (c): Chromatograms of sequencing results viewed by Finch TV software show nucleotide variations in 16S rRNA gene of H. pylori illustrated by colored boxes and arrows. (b): Multiple Sequence Alignment (MSA) of 16S rRNA sequences of three Sudanese H. pylori strains compared with rRNA gene of other selected strains obtained from GenBank databases using Clustal W2.
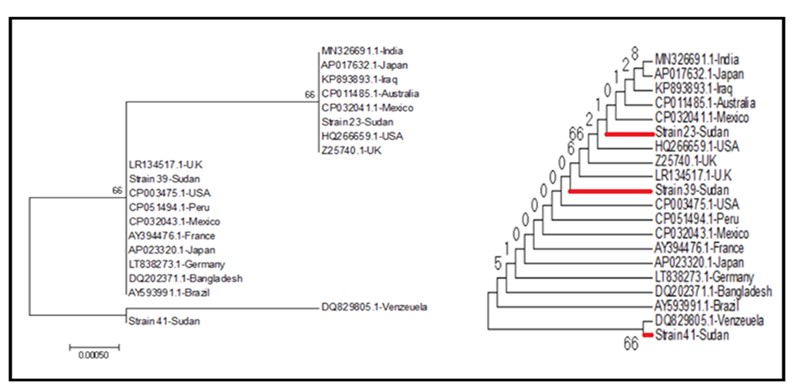
Figure 4. The neighbor-joining tree for the H. pylori 16s rRNA gene of 3 Sudanese strains compared with 17 global reference strains from the GenBank database. The percentage of replicate trees (1000 replicates) is shown next to the branches. The evolutionary distance was computed using JC method and is in the units of the number of base substitutions per site. Evolutionary analyses were conducted using MEGA7.
The in vitro antimicrobial susceptibility of each EO was estimated quantitatively by MICs and minimum bactericidal concentrations (MBCs) (Table 2). The results showed that the entire six EOs completely inhibited the growth of all selected H. pylori clinical isolates at concentration of 125 µg/ml. Clove and maharaib oils exhibited maximum activity and inhibited the growth of all strains even at concentration of 3.9 µg/ml. The other EOs showed no bactericidal effect at this concentration.
A significant difference (P=0.00) was detected in activities of EOs from different plant families. The second potent EOs were cinnamon, thyme and lemongrass, which showed the same MIC and MBC value of 15.625 µg/ml. Frankincense oil exhibited the highest MIC value of 125 µg/ml and of course the least activity against all tested strains (Table 2).
Determination of EOs Synergism Against H. pylori
At the highest concentration (125 µg/ml) all EO combinations demonstrated bactericidal activity against all tested strains without exception. Regarding MICs; the combinations of cinnamon and maharaib oils, thyme and maharaib oils, frankincense and maharaib oils, and lemongrass and maharaib oils showed the highest inhibitory effect with MIC value of 0.97 µg/ml. Clove and cinnamon oils combination showed an increase in antibacterial activity as compared to each of them separately. Multivariate analysis showed significant differences between inhibitory effects of EOs combinations (P=0.00) on H. pylori strains.
According to FIC indices; our results indicated synergistic activity in 33.3% (5/15) of the combinations (Table 3). Five synergistic combinations, which exhibit MICs of 0.97 µg/ml and 3.9 µg/ml and FIC indices from 0.26 to 0.5 were: cinnamon and lemongrass oils, cinnamon and maharaib oils, thyme and maharaib oils, frankincense and maharaib oils, and lemongrass and maharaib oils. The combination of frankincense oil and Maharaib oil was found to possess the best synergistic effect with FIC index of 0.26 (Figure 5). Moreover, clove EO displayed no synergistic effect with other studied essential oils. Interestingly, the combinations of thyme EO with other oils resulted in antagonistic effect except when mixed with maharaib and cinnamon oils. Nevertheless, antagonistic effect was also observed when combining frankincense oil with thyme, lemongrass, clove, and maharaib oils.
Table 2. MICs and MBCs of EOs against H. pylori clinical strains
| Essential oils Common name |
Family/Botanical name | Extracted part | Mean MICs/ MBCs µg/ml |
| Frankincense | Burseraceae Boswelliapapyrifera (Delile ex caill.) Hoshst. |
Resin | 125 |
| Thyme | LamiaceaeThymus vulgaris L | Aerial parts | 15.625 |
| Cinnamon |
Lauraceae. CinnamomumverumJ.S. Presl | Barks |
15.625 |
| Clove |
Myrtaceae Syzygiumaromaticum (L.) Merr. & L.M.Perry |
Flower buds | 3.9 |
| Lemongrass | Poaceae (Graminae) Cymbopogoncitratus (DC.) Stapf |
leaves | 15.625 |
| Camel'shay (Maharaib) | Poaceae Cymbopogonschoenanthus (L.) Spreng ssp. proximus |
Root | 3.9 |
Table 3. Susceptibility of H. pylori clinical isolates to EOs in combination blends (1:1)
| EOs | Mean MICs/ MBCs (1.1) µg /ml |
FIC of essential oil 1 µg /ml | FIC of essential oil 2 µg /ml | FIC index | Interaction |
| AB | 3.9 | A = 1 | B = 0.24 | 1.24 | Additive |
| AC | 15.625 | A= 4 | C= 1 | 5 | Antagonism |
| AD | 125 | A= 32 | D= 1 | 33 | Antagonism |
| AE | 15.625 | A= 4 | E= 1 | 5 | Antagonism |
| AF | 3.9 | A= 1 | F= 1 | 2 | Additive |
| BC | 15.625 | B= 1 | C= 1 | 2 | Additive |
| BD | 15.625 | B= 1 | D=0.13 | 1.13 | Additive |
| BE | 3.9 | B= 0.25 | E= 0.25 | 0.5 | Synergism |
| BF | 0.97 | B= 0.06 | F= 0.25 | 0.31 | Synergism |
| CD | 125 | C= 8 | D= 1 | 9 | Antagonism |
| CE | 125 | C= 8 | E= 8 | 16 | Antagonism |
| CF | 0.97 | C= 0.06 | F= 0.25 | 0.31 | Synergism |
| DE | 125 | D= 1 | E= 8 | 9 | Antagonism |
| DF | 0.97 | D= 0.01 | F= 0.25 | 0.26 | Synergism |
| EF | 0.97 | E = 0.06 | F= 0.25 | 0.31 | Synergism |
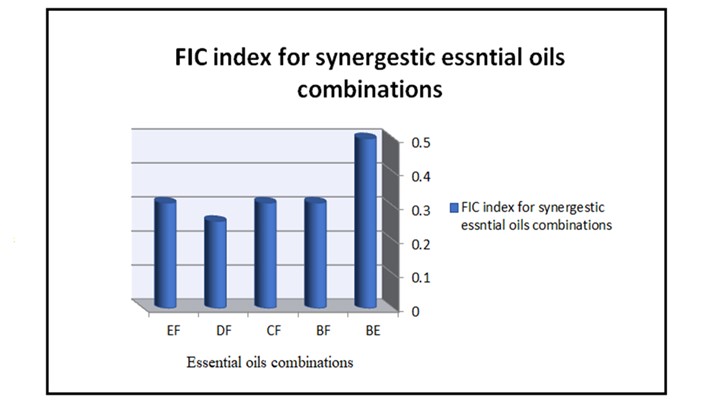
Figure 5. Synergistic EOs combinations. (EF=Lemongrass oil-Maharaiboil, DF Frankincense oil-Maharaiboil, CF=Thyme oil-Maharaiboil, BF=Cinnamon oil-Maharaib oil, BE=Cinnamon oil-Lemongrass oil. FIC=Fractional inhibitory concentration).
In this study, we utilized conventional culturing, PCR, and sequencing methods for identification of H. pylori. The analysis of the obtained 16S rRNA sequences further confirmed H. pylori identification of our isolates; however, noticeable level of genetic diversity between isolates and various sequences has been observed.
Understanding the genetic relationships and variations among H. pylori strains is vital for the public health strategies. Moreover, phylogenetic analysis revealed sources of infection and transmission routes, which in turn guides treatment decisions. Our results showed that two lineages diverge from un-rooted phylogenetic tree. Lacking the root emphasizes links among strains, rather than their ancestry. This is particularly relevant when the evolutionary history is not completely understood. Moreover, two major clades in phylogenetic tree showed significant genetic differences among strains. These differences are likely the result of historical migrations and local adaptations. Consequently, recognizing such differences help in understanding variations in virulence as well as disease outcomes (39-42).
Furthermore, the clustering of Sudanese strains with those from Mexico and Australia in one lineage suggests they share evolutionary pressures. These may be due to similar environments or diets, which could affect the spread of H. pylori infections in these regions. Regarding the other linage, Strain 41-Sudan exhibits a longer branch, which suggests unique evolutionary pressures or adaptations. Our findings are consistent with a previous study that characterized H. pylori strains in Sudan (7, 40-42).
The biological activity of plant EOs against H. pylori strains has been previously established, however; in this study markedly variable bactericidal activity was observed among EOs. This variability is probably due to various reasons, for instance; even within one species, the MIC values of EOs chemo-types might sometimes differ, particularly within a chemo-type if different strains of the same microorganism were used. Additionally, using the same plant sourced from different suppliers or employing various methods may influence the obtaining results (10, 37, 38, 43, 44). The present study showed the potency of EOs of C. schoenanthus (maharaib), S. aromaticum (clove), C. citratus (lemongrass), C. verum (cinnamon), T. vulgaris (thyme) and B. papyrifera (frankincense) and their combinations against specific H. pylori clinical isolates (MW599345, MW599346 and MW599347, MZ813315, MZ813316) originated from human gastric samples.
The findings of the current study align with the literature, which also confirmed the superior antibacterial activities of maharaib oil in comparison with other oils against H. pylori reference strain (10). Nevertheless, the different MIC values obtained probably attributed to different geographical origin of C. schoenanthus oil as well as different extraction method.
Although the results revealed by Korona-Glowniak et al (10) proved anti H. pylori proprieties of clove EO, they found a lower bactericidal activity with MIC of 31.3 mg/L. This variation is not surprising, as they used different H. pylori strains. Additionally, the antibacterial activity of T. vulgaris EO against H. pylori has been confirmed by numerous studies (10, 38, 44). The MIC for thyme oil was obtained 15.6 µg/ml and it was exactly the same as what found in a Poland study (45).
On the other hand, current result is opposed to those previously determined in several studies worldwide. For instance, the effect of thyme EO on viability of H. pylori strain was determined in a liquid medium and it was found to be very effective with a very low MBC at 0.4 µg/ml (38). Current finding also differs from that of Esmaeili et al (44) who recorded a lower MBC value against standard strain of H. pylori. This discrepancy may be attributed to the use of different plant parts from various habitats, as well as the use of different media and bacterial strains.
Moreover, Bergonzelli et al (38) reported a similar finding; all H. pylori strains tested in their study were equally sensitive to C. citrates and C. verum EOs.
Furthermore, several studies have investigated the antibacterial activity of Betula papyrifera EO against different pathogens (20, 26). However, to the best of our knowledge, the current study is the first to investigate the bactericidal activity of frankincense EO against H. pylori clinical strains.
Regarding cinnamon, several studies have shown that cinnamaldehyde, a compound in cinnamon, possesses strong urease inhibition activity, which may explain the anti-H. pylori properties of cinnamon EO (46, 47).
We investigated for the first time the antibacterial efficacy of the above mentioned synergistic blends against H. pylori clinical strains. The bioactivity of mixed EOs is still not completely understood, however, synergism results in affecting multiple target sites in the bacterial cell as diverse components of oils may act synergistically giving rise to the efficiency of the whole oils interactions (48). It has been reported that the major constituents in cinnamon oil that are responsible for its antimicrobial potency are cinnamldehyde, eugenol, and linalool (48). Moreover, EOs containing citral or eugenol as major components exert high antibacterial activity (48). Citral, in particular, is reported to disrupt bacterial cell membranes and interfere with cellular functions (49). It is noteworthy that the combination of essential oils from Cymbopogon species like C. citrates and C. schoenanthus exhibited strong anti-H. pylori effects, likely due to the presence of citral in both oils. Similarly, the mixture of C. citrates and C. verum EOs were more effective than individual oils. The main possibility is that cinnamaldehyde, as a component of cinnamon oil, strengthened the effects of citral on disrupting the bacterial cell membrane. As a result, the combination of cinnamon with C. schoenanthus EOs exhibited a remarkable synergism due to cinnamaldehyde effect together with citral in the enrichment of their antibacterial activity (46, 49).
The high antimicrobial activity of Thymus species has been attributed to their phenolic components such as thymol and carvacrol (48). Combining thyme and C. schoenanthus EOs revealed promising synergistic antibacterial effects, where thymol disrupts bacterial membranes and combines with carvacrol to act on different microbial targets (48, 50). Moreover, the combination of frankincense and C. schoenanthus EOs showed synergistic inhibition of H. pylori, where boswellic acids in frankincense EO may enhance the antibacterial properties of the components in C. schoenanthus (51).
Various studies addressed synergism between EOs components, however, additive and even antagonistic effects among different compounds have also been observed. For instance, mixing eugenol with linolool has been reported to possess synergistic interaction (37) and that might explain to some extent the low FIC value obtained in the current study from cinnamon-clove combination.
Furthermore, the present study showed the potency of maharaib oil in enhancing the antibacterial activity of the other tested oils, yet the reason of this enhancement is unknown. Numerous studies acknowledged that the chemical composition of maharaib oil from Sudan contains piperitone, carvacrol and thymol (52). Piperitone is the major compound of C. schoenanthus oil and it has been already addressed to synergize the antibacterial activity of some antibiotics (52, 53). However, it has been assumed that not only the major components are responsible for the total activity of EOs, but also components of lower abundance should be taken into account (54). That is why the combination of whole EOs unexceptionally has higher antibacterial activity than the mixtures of their major components (50, 55). Generally, these synergistic effects are attributed to mechanisms such as enhanced cell membrane disruption, enzyme inhibition, and interference with bacterial communication pathways. Further in vivo studies are necessary to confirm the efficacy and therapeutic potential of these EO combinations against H. pylori infections.
The narrow scope of this study, which utilized only six essential oils from Sudan, coupled with limited sample size, constrains the generalizability of the findings. Yet it reveals promising insights into essential oils as potential H. pylori treatments, especially in regions where antibiotic resistance is a growing concern. The absence of in vivo trials limits definitive conclusions about the oils efficacy and synergistic effects. However, previous research has demonstrated in vivo anti-H. pylori activity for several tested oils (9). Despite the current limitations, these results lay groundwork for the future in vivo studies and possible therapeutic developments.
The obtained results pointed out the great potential of S. aromaticum and C. schoenanthus oils to prevent the in vitro growth of the H. pylori strains. Moreover, the synergistic combinations of cinnamon-lemongrass, cinnamon-maharaib, thyme-maharaib, frankincense-maharaib and lemongrass-maharaib displayed promising alternative therapeutic agents targeting H. pylori infection. Furthermore, essential oil of C. schoenanthus strengthened the efficacy of other essential oils under study.
Authors would like to thank Dr. Hatim Mudawi and the nursing staff at Gastrointestinal Tract Endoscopy Unit at Soba University Hospital. Sincere thanks as well to Miss Abeer Babiker for her great help during sample collection. Many thanks and gratitude to the assistance received from Dr. Hassan Hussein, Miss Arwa El-Aqib, Miss Safa Mohammed Elhassan, FMLS, University of Khartoum. Dr. Khalid A. Enan and Miss Tarteel Hassan, in the Virology Department Central lab are acknowledged as well for their generous assistance. The authors love to thank Dr. Yahia Suliman in Medicinal and Aromatic Plants and Traditional Medicine Research Institute (MAPTMRI) for his ultimate support.
Ethical Considerations
Not applicable.
Authors’ Contributions
All authors have reviewed the final version to be published and agreed to be accountable for all aspects of the work.
1- Maryam A SalahEldin: Concept and design, Acquisition, analysis, or interpretation of data, Drafting of the manuscript, Critical review of the manuscript for important intellectual content. 2- El-Amin Mohamed Ibrahim: Acquisition, analysis, or interpretation of data, Critical review of the manuscript for important intellectual content, Supervision. 3- Sara Gamal Gubara Mohamed: Acquisition, analysis, or interpretation of data, Drafting of the manuscript. 4- Samah Awad AbduRahim, 5- Hadeel Gassim Hassan, 6- Ali Elbagir Ali Mohamed, 7- Leena Babiker Idris Babiker, 8- Hana A Elkhalifa, 9- Marwa M Suliman, 10- Marwan M Badawi, and 11- Mohamed A. Hassan: Concept and design, Drafting of the manuscript. 12- Aisha Zoheir Ibrahim Almagboul: Concept and design, Drafting of the manuscript, Critical review of the manuscript for important intellectual content, Supervision.
All authors have declared that they have no financial relationships with any organizations that might have an interest in the submitted work.
Conflicts of Interest
Received: 2024/08/18 | Accepted: 2024/12/9 | ePublished: 2025/01/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |