BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2291-en.html


 , Iraj Nikokar2
, Iraj Nikokar2 

 , Ali Mojtahedi3
, Ali Mojtahedi3 

 , Mohammadreza Mobin4
, Mohammadreza Mobin4 
 , Zahra Atrkar Roshan1
, Zahra Atrkar Roshan1 
 , Moslem Karampour1
, Moslem Karampour1 

2- Department of Microbiology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran & Medical Biotechnology Research Center, Laboratory of Microbiology and Immunology of Infectious Diseases, Paramedicine Faculty, Guilan University of Medical Sciences, Rasht, Iran ,
3- Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
4- Department of Surgery, Guilan University of Medical Science, Rasht, Iran
Pseudomonas aeruginosa is an opportunistic pathogen that poses a significant threat to patients with underlying conditions, such as respiratory infections like cystic fibrosis and burn wound infections (1). In fact, this bacterium is the leading cause of death in patients admitted to the burns ward (2-4). However, the increasing resistance of P. aeruginosa to a wide range of antibiotics in recent years has made treating infections in burn wound patients a significant challenge (5, 6). The excessive use of antibiotics during treatment has accelerated the development of multidrug-resistant (MDR) P. aeruginosa strains, which can counter most antibiotics using different intrinsic and acquired resistance mechanisms. P. aeruginosa exhibits a high level of intrinsic resistance to certain antibacterial agents due to decreased outer membrane permeability, efflux systems that pump antibiotics out of the cell, and the production of enzymes that inactivate antibiotics (5-7). Acquired resistance of P. aeruginosa can be achieved through either horizontal transfer of resistance genes or mutational changes (8). Fluoroquinolones are an important class of antibiotics widely used in the treatment of Pseudomonas infections due to their effective antimicrobial activity, tissue diffusion, and accessibility in oral form. This group of antibiotics acts as inhibitors of DNA gyrase and topoisomerase IV, which are essential for bacterial DNA transcription and replication. These enzymes are heterotetrameric with two subunits, gyrase being constituted as gyrA and gyrB and topoisomerase IV as parC and parE. The mechanisms of fluoroquinolone resistance in P. aeruginosa are mainly due to point mutations in DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) within the Quinolone-Resistance-Determining Regions (QRDR) (9). Fluoroquinolone resistance can also occur through a novel mechanism called plasmid-mediated quinolone resistance (PMQR), which can be transmitted between P. aeruginosa bacteria and facilitate the spread of this resistance (10, 11). Therefore, determining the patterns and mechanisms of antibiotic resistance in P. aeruginosa is crucial in selecting an effective approach to managing and preventing burn wound infections (12). In this study, we analyzed mutations of gyrA, gyrB, parC, and parE genes in the QRDR of P. aeruginosa isolated from patients with burn wound infections.
Bacterial isolates
A total of 300 samples were gathered from patients hospitalized with wound burn infections at the Velayat Educational Burn Center in Rasht, Guilan, Iran, spanning the years 2016 to 2019. These samples were then transferred to tryptic soy broth (TSB) from Merck, Germany, and cultured on cetrimide agar medium to isolate P. aeruginosa. The identification of the isolates was carried out using standard biochemical tests (13). Approval for this study was granted by the Ethical Committee of Guilan University of Medical Sciences under ethical code IR.GUMS.REC.1397.284.
Antibiotics susceptibility testing
The agar disk diffusion method was employed to conduct antibiotic susceptibility tests in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (14). The following antimicrobial agents were utilized to determine the antibiotic susceptibility of P. aeruginosa: Amikacin (30ug), Gentamicin (10ug), Tobramycin (10ug), Ceftazidime (30ug), Imipenem (10ug), Piperacillin (100ug), and Ciprofloxacin (5ug) (Mast Diagnostics, Mast Group Ltd, Merseyside, UK). The antibiogram test utilized the standard strain of P. aeruginosa ATCC 27853 as a positive control. MDR isolates were defined as those resistant to three or more antibiotic classes (15, 16).
DNA extraction
The genomic DNA of P. aeruginosa isolates was extracted using a Genomic DNA purification Kit (Roche, Mannheim, Germany) following the manufacturer's instructions. The quantity and quality of the extracted DNA were assessed at OD 260/280 nm and on a 1.5% agarose gel. The extracted DNA was then stored at -80°C.
Polymerase chain reaction (PCR) amplification
PCR amplification was carried out utilizing the primer sets detailed in Table 1. The DNA amplification process involved an initial denaturation step at 94°C for 2 minutes, followed by 35 cycles comprising denaturation at 94°C for one minute, annealing at 60°C for 30 seconds, extension at 72°C for one minute, and a final extension at 72°C for five minutes using a thermocycler device (Eppendorf Mastercycler Gradient, Germany). The amplified DNA products were then analyzed through electrophoresis on a 1% agarose gel (17, 18).
DNA sequencing
The purified PCR products were sequenced utilizing the Applied Biosystems 3730/3730xl DNA analyzers sequencing (ABI) system from Bioneer Co., Korea. Subsequently, the sequence of each sample was compared to the P. aeruginosa PAO1 sequence using online BLAST software (http://www.ncbi.nlm.nih.gov/BLAST/).
Table 1. Characteristics and sequence of primers used for molecular techniques in P. aeruginosa isolates
| Size (bp) | Primer sequence | Primer | Gene |
324 |
AGTCCTATCTCGACTACGCGAT | F | gyrA |
| AGTCGACGGTTTCCTTTTCCAG | R | gyrA | |
483 |
TGCGGTGGAACAGGAGATGGGCAAGTAC | F | gyrB |
| TGGCGGAAGAAGAAGGTCAACAGCAGGG | R | gyrB | |
282 |
CGAGCAGGCCTATCTGAACTAT | F | parC |
| GAAGGACTTGGGATCGTCCGGA | R | parC | |
564 |
CGGCGTTCGTCTCGGGCGTGGTGAAGGA | F | parE |
| TCGAGGGCGTAGTAGATGTCCTTGCCGA | R | parE |
Bacterial isolates
Out of the 300 patients admitted to the Burn Center of Guilan University of Medical Sciences (Velayat Burns Center, Rasht), 118 P. aeruginosa isolates were identified, accounting for 39.3% of the total. Among these isolates, 37 (31.35%) were detected in female patients, while 81 (68.64%) were found in male patients. The distribution of isolates was assessed across various age groups, revealing the highest occurrence in the 21-40 age bracket (39.83%) and the lowest occurrence in age groups under 20 years and between 61-90 years (16.10%).
Antibiotics resistance in P. aeruginosa
In terms of antibiotic resistance in P. aeruginosa, high levels of resistance were observed for Tobramycin (59.32%), followed by Gentamicin (55.08%) and Piperacillin (51.69%). The lowest rate of resistance was detected in Imipenem (23.72%), Amikacin (26.27%), and Ceftazidime (30.50%). Out of the 118 isolates, 42 (35.59%) were classified as MDR due to their resistance to at least one antibiotic from three antibiotic classes. Among the 118 isolates, 60 (50.84%) were resistant, 8 (6.77%) were intermediate, and 50 (42.37%) were susceptible to Ciprofloxacin. The highest intermediate resistance was observed with Imipenem, with 23 (19.49%) isolates exhibiting intermediate resistance to this antibiotic. The details of the antimicrobial resistance pattern results of P. aeruginosa isolates are presented in Table 2.
Analysis of the mutations in QRDR
To analyze mutations in the QRDR genes, the extracted DNA from all P. aeruginosa isolates was amplified for the gyrA, gyrB, parC, and parE genes using PCR (Figure 1). The quality and quantity of PCR products were assessed on an agarose gel, and 35 samples were chosen for sequencing. Among the 35 clinical isolates, 20 strains exhibited resistance to Ciprofloxacin, while 15 strains displayed susceptibility to Ciprofloxacin. QRDR mutations were identified in 15 out of the 35 strains. Fourteen strains exhibited mutations in the gyrA gene, one mutation was found in parE, and no mutations were detected in the gyrB and parC genes. Regarding gyrA, 12 (85.71%) of the strains showed a missense mutation of Threonine (ACC) Isoleucine at the 83rd codon (Thr 83 Ile). All isolates were resistant to Ciprofloxacin. Among these 12 strains, 5 exclusively displayed the Thr 83 Ile mutation (group I). In two isolates, along with the Thr 83 Ile mutation, silent mutations were also present (group II). Other mutations were categorized into different groups: Thr 83 Ile with Thr-141 Ala (Alanine) and Asp-143 Asn (Asparagine) mutations (group III) in 5 strains, and in one strain, 3 mutations (Asp-87 Asn, Thr-132 Met (Methionine), and Lys-141 Arg (Arginine) with Thr 83 Ile). Two silent mutations in gyrA were observed in two ciprofloxacin-sensitive isolates (group IV). In the case of the parE gene, a mutation at codon 470 (Asp replaced by Asn) was detected in a Ciprofloxacin-resistant strain. The details of the mutations occurring in the QRDR genes of P. aeruginosa isolates are presented in Table 3.
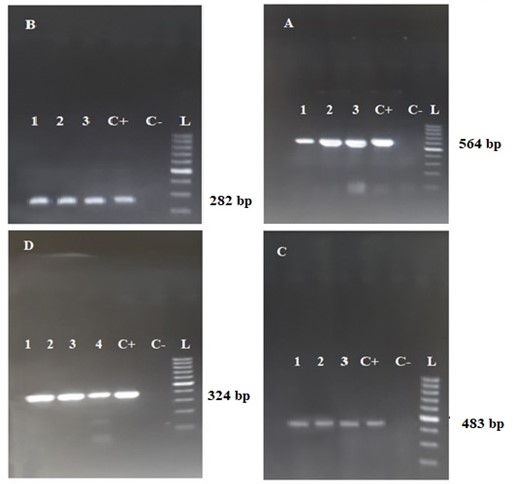
Figure 1. The figure A, B, C, and D are detected in the agarose gel, with bands corresponding to parE, parC, gyrB, and gyrA, respectively. Wells 1 to 4 represent the clinical samples, while C+, C-, and L denote the positive control, negative control, and molecular size marker (ladder), respectively.
Table 2. Antimicrobial pattern of P. aeruginosa isolates
| Antimicrobials | Resistant (R) | Intermediate (I) | Sensitive (S) | |||
| Isolates | Rate (%) | Isolates | Rate (%) | Isolates | Rate (%) | |
| Ciprofloxacin | 60 | 50.84 | 8 | 6.77 | 50 | 42.37 |
| Gentamycin | 65 | 55.08 | 5 | 4.23 | 48 | 40.67 |
| Amikacin | 31 | 26.27 | 16 | 13.55 | 71 | 60.16 |
| Tobramycin | 70 | 59.32 | 10 | 8.47 | 38 | 32.20 |
| Ceftazidime | 36 | 30.50 | 10 | 8.47 | 72 | 61.01 |
| Piperacillin | 61 | 51.69 | 10 | 8.47 | 47 | 39.83 |
| Imipenem | 30 | 25.42 | 23 | 19.49 | 66 | 55.93 |
Table 3. Mutation groups detected in the gyrA and parE genes of P. aeruginosa isolates
| Sensitive | Resistant | Others mutations | Non sense Mutation |
Dominant Mutation |
Groups of Mutation gyrA Gene |
| - | 5 | ------- | -------- | ACC →ATC (Thr-83→ Ile) |
I |
| - | 2 | ------ |
CAC→CAT
(His-132→His) GAA→GAG (Glu-141→Glu)
|
ACC →ATC (Thr-83→ Ile) |
II |
| - | 5 | GAC→AAC Asp144→Asn (2) ACC→GCC (Thr-142→Ala) (2) GAC→AAC (Asp-87→Asn) |
---------------- | ACC →ATC (Thr-83→ Ile) |
III |
| 2 | - |
|
CAC →CAT
(His-132→His) GAA→GAG (Glu-141→Glu)
|
---------- | IV |
| - | 1 | ----------------- | ------------------ | GAT→AAT (Asp-470→Asn) |
ParE |
P. aeruginosa is a significant opportunistic pathogen known for causing healthcare-associated infections (HAIs) and outbreaks in patients with burn wounds. In recent years, there has been an increase in resistance to various antimicrobial agents among P. aeruginosa isolates (19, 20). This study identified 118 (39.33%) P. aeruginosa strains from samples of patients with burn wound infections. Our previous research indicated a decrease in the frequency of P. aeruginosa isolates to 47% in the burn center (12). Furthermore, we observed that P. aeruginosa isolates showed the lowest resistance to Imipenem, consistent with our initial study (12). Corresponding to our findings, multiple global studies have emphasized P. aeruginosa as a predominant bacterium isolated from burn wounds, presenting challenges in treating burn patients due to its antibiotic resistance (21-23). Nevertheless, resistant strains to Imipenem, previously considered effective in many studies, are on the rise in burn centers (24). Tarafdar et al.'s research also recognized Imipenem as the most effective antibiotic against P. aeruginosa isolated from burn wounds, aligning with our results (21). Conversely, Tchakal-Mesbahi et al. found that 100% of P. aeruginosa isolates from burn patients' wounds in Algeria were resistant to Imipenem (23). The variation in Imipenem resistance levels among P. aeruginosa isolates may be attributed to geographical differences and the antibiotic's usage in clinical settings. Regarding MDR isolates, 42 (35.59%) of the isolates were classified as MDR, a decrease from our previous study. Kishk et al. reported a 70% rate of MDR P. aeruginosa isolates from burn wounds in Egyptian patients, which is higher than our findings (22). Ahmadian et al. reported a 54.76% frequency of MDR isolates in clinical samples of P. aeruginosa, indicating potential variations based on sample type and geographical location (25). The inherent resistance of P. aeruginosa to several antibiotics is due to factors such as low permeability of the outer membrane, constant efflux pump expression, and production of antibiotic-inactivating enzymes (26). Common antibiotics used in treating infections caused by P. aeruginosa include beta-lactams, aminoglycosides, and fluoroquinolones (27). The indiscriminate use of antibiotics and mutations in P. aeruginosa genes can lead to antibiotic resistance in this bacterium (28). Alterations in the QRDR of DNA gyrase and topoisomerase IV are the primary mechanisms of fluoroquinolone resistance in P. aeruginosa (29). In this study, 35 isolates were sequenced for QRDR genes, encompassing gyrA, gyrB, parC, and parE genes. Fourteen (87.5%) of the strains exhibited mutations in the gyrA gene. Akasaka et al.'s research on the QRDR region of P. aeruginosa (30) also highlighted the high frequency of mutations in the gyrA gene, consistent with observations from other studies by various researchers (10, 31, 32). The findings of this study reveal that the most prevalent mutation in the gyrA gene is Thr83Ile, resulting in the substitution of threonine with isoleucine at codon 83. Consistent with our results, several studies have indicated that Thr83Ile mutations are the most common type of mutation occurring in the gyrA gene (33-35). Mutational changes were evaluated as an acquired mechanism of resistance to fluoroquinolones. The most common substitution observed was threonine replaced by isoleucine at codon 83, as reported in various studies, indicating that plasmid transfer of this mechanism might contribute to the high prevalence of resistance seen in the study (33-35). In Iran, a study found that nearly 90% of resistant P. aeruginosa isolates had a gyrA mutation, with the predominant mutation being threonine to isoleucine at codon 83 (34). Additionally, two isolates exhibited silent mutations in the gyrA gene, associated with Ciprofloxacin-sensitive strains. Variability in mutations within this gene was noted in five strains, showing not only the prevalent Thr 83 Ile mutation but also other mutations. Similar scenarios have been documented in studies conducted by Wang et al. in Taiwan (19), Wydmuch et al. in Poland (36), and Akasaka in Japan (30). No mutations were found in the quinolone resistance-determining regions (QRDRs) of the gyrB and parC genes. However, a mutation was identified in the parE gene, involving the substitution of aspartic acid with asparagine (Asp-470→Asn) in the Ciprofloxacin-resistant strain. This mutation differed from those observed by Wang et al. and Akasaka et al. in Ciprofloxacin-resistant strains. Akasaka et al. highlighted that the main alterations linked to fluoroquinolone resistance were the replacement of Thr-83 with Ile in GyrA and Ser-87 with Leu in ParC. Wang et al. noted various amino acid substitutions in the parC gene, such as Gly85Cys, Ser87Leu, Ser87Trp, and Glu91Lys, in fluoroquinolone-resistant strains (19, 30). In the study, a primary mechanism of resistance to fluoroquinolones in P. aeruginosa isolates was demonstrated. However, limitations included a focus solely on mutations related to this resistance mechanism without exploring other mechanisms or underlying reasons for these mutations. To gain a comprehensive understanding of antibiotic resistance against fluoroquinolones, future research should investigate each mechanism—efflux pumps, biofilm formation, and reduced drug penetration—alongside chromosomal mutations. This integrated approach will help determine if these mechanisms coexist simultaneously.
The results of this investigation revealed that mutations in the gyrA gene are the primary mechanism driving fluoroquinolone resistance, particularly against Ciprofloxacin. Specifically, the most frequent mutation occurs at the Thr 83 Ile position, where changing the amino acid's polarity can impede the function of the DNA gyrase enzyme. To combat the spread of fluoroquinolone-resistant P. aeruginosa isolates, it is crucial to both reduce the extensive use of fluoroquinolones and accurately identify fluoroquinolone-resistant isolates along with their underlying mechanisms. Subsequently, novel therapeutic strategies could be implemented.
No to declared .
Ethical Considerations
No to declared .
Authors’ Contributions
F.A. and A.M. designed the topic and wrote the manuscript. I.N. and A.M. and M.M. participated in the initial draft and the revision of the manuscript. Z.A.R. and M.K. revised the final version of the manuscript. All authors read and approved the final manuscript.
This study was supported by Guilan University of Medical Sciences, Grant No. P/3/132/4266.
Conflicts of Interest
The authors declare that they have no competing interests.
Received: 2024/01/8 | Accepted: 2024/05/10 | ePublished: 2024/05/25
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





