BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2250-en.html


 , Hamideh Najafi1
, Hamideh Najafi1 

 , Zahra Ziafati Kafi1
, Zahra Ziafati Kafi1 

 , Seyed Mohammad Ali Hashemi2
, Seyed Mohammad Ali Hashemi2 

 , Jamal Sarvari2
, Jamal Sarvari2 

 , Arash Ghalyanchi Langeroudi3
, Arash Ghalyanchi Langeroudi3 


2- Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
3- Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran ,
Glioblastoma, formerly referred to as glioblastoma multiforme (GBM), stands as one of the most aggressive forms of brain cancer, marked by symptoms including headaches, personality alterations, nausea, and stroke-like manifestations (1). The onset of these symptoms is typically rapid and can progress to unconsciousness (1). While the exact causes of GBM and other gliomas remain elusive, there is a documented heightened risk in a small subset of GBM patients exposed to ionizing radiation, chemical agents, or genetic predispositions (2). Recently, greater attention has been focused on the potential viral origins of gliomas, as they may act as oncomodulators, with viral proteins capable of augmenting neoplastic processes through the disruption of various intracellular signaling pathways (3, 4). Epstein-Barr virus (EBV) is alternatively known as HHV-4 and holds the distinction of being the first recognized human oncovirus (5). While B-cells in the bone marrow are commonly considered the primary latent reservoir of EBV, their presence has also been detected within the brain (7). Several central nervous system (CNS) disorders, such as acute encephalitis, acute cerebellar ataxia, demyelinating diseases, myelitis or meningitis, and other CNS neuropathies, have been suggested to have potential links to EBV infection (8). Complement receptor 2 (CR2), recognized as the primary cellular receptor for Epstein-Barr virus (EBV), has been identified in astrocytes, facilitating the virus's entry into astrocyte cell lines and subsequently promoting increased proliferation (9, 10). Significantly, EBV is frequently detected in primary CNS lymphomas, particularly diffuse large B-cell lymphomas and lymphoid granulomatosis (11). Within the realm of EBV proteins, EBV nuclear antigen-1 (EBNA1) stands out as the lone protein found across all EBV latency types (12). EBNA1 plays a pivotal role in the regulation of transcription for both viral and cellular promoters (12). Functioning as a DNA-binding transcription factor, this protein interacts with diverse DNA regions within the cellular genome, including the promoters of genes whose transcription is under the influence of EBNA1 (13, 14).
In this study, the first pair of cellular genes scrutinized were mouse double minute X homolog (MDMX) and MDM2. These genes produce proteins that are capable of binding to the p53 tumor suppressor protein, effectively inhibiting its function. Notably, both MDMX and MDM2 have been observed to be overexpressed in numerous human cancer types (15). However, it's important to distinguish their mechanisms: MDM2, which is a nuclear-localized E3 ubiquitin ligase, directly degrades p53, whereas the MDMX protein inhibits p53 by binding to its transcriptional activation domain. Additionally, MDMX forms an interaction with MDM2 through the RING finger domain, preventing the degradation of the latter (16). On a different note, MYC, functioning as a transcription factor, exerts influence over a range of biological processes, including cell growth, proliferation, apoptosis, and cellular metabolism (17). Meanwhile, BIRC5, by interfering with caspase activity, mitigates cell death, and thus, its elevated expression has been linked to various cancer types (18, 19).
Building upon the diverse characteristics of EBV-EBNA1, our novel research endeavored to assess the impact of EBNA1 on the expression profiles of four critical cellular oncogenes associated with glioblastoma (GBM). Specifically, we focused on MDMX, MDM2, MYC, and BIRC5. To unravel this intricate interplay, we employed the U87MG glioblastoma astrocytoma cell line, which has been genetically modified through transfection with a plasmid carrying the EBNA1 gene.
Plasmid Preparation and Validation, and Bacterial Transformation
In this research endeavor, we utilized the pCEP4 plasmid (Thermo Fisher Scientific, USA), which is an EBV-based plasmid containing the EBNA1 gene alongside the hygromycin B resistance gene, in addition to pcDNA3.1/hygro as control plasmid devoid of the EBNA1 gene which was obtained from the Iranian Biological Resource Center (IBRC). These plasmids were individually transformed and propagated within the Escherichia coli DH5α strain. To confirm the presence of the EBNA1 gene in the pCEP4 plasmid, we conducted enzyme digestion and colony PCR. Subsequently, both the recombinant and control plasmids were separately extracted utilizing the MBST plasmid isolation kit (Molecular Biological System Transfer, Tehran, Iran), and their quality and concentrations were assessed through gel electrophoresis and spectrophotometry.
Cell Culturing, Transfection, and Selection of Transfected Cells
U87MG cells, a widely employed human glioblastoma cell line in brain cancer research, were acquired from the IBRC and cultivated in Dulbecco's Modified Eagle Medium (DMEM; Bioidea, Tehran, Iran) supplemented with 10% Fetal Bovine Serum (FBS; Gibco, USA) under standard conditions of 37°C and 5% CO2 within a 6-well plate. Once the cells reached an approximate confluency of 80%, they were subjected to transfection using an optimized concentration of plasmids (4 µg) and DNA-fectamine (6 µL; BioBasic, Canada). One set of cells received the recombinant plasmid carrying the EBNA1 gene, while the other group was transfected with a mock plasmid lacking the EBNA1 gene. Following transfection, both cell populations underwent selection using 500 μg/mL hygromycin B (BioBasic, Canada). To attain complete cell selection, transfected cells were maintained under stable hygromycin B concentrations over several passages for approximately 25 days.
RNA Extraction, cDNA Synthesis, Real-time PCR, and Data Analysis
The total RNA from both cell groups was extracted using an RNA Isolation Kit (Dena Zist, Iran). The quantity and quality of the RNA from each group were assessed through spectrophotometry and electrophoresis, respectively. To ensure the exclusion of plasmid contamination, RNase-free DNase (Sinaclon, Tehran, Iran) was employed. Subsequently, a cDNA Synthesis Kit (Dena Zist, Iran) was utilized to reverse-transcribe the extracted RNA from each sample into stable and optimized concentrations of cDNA. Real-time PCR based on SYBR green fluorescence was then conducted using specific primers to measure the expression of the chosen cellular genes. For relative quantification of gene expression, the beta-actin gene was used as a reference gene. In each 20 μL final volume mixture, there were 10 μL of 2x SYBR Green Master Mix (Ampliqon Inc., Denmark), 7 μL of molecular grade water, 0.5 μL of each specific primer pair (refer to Table 1), and 2 μL of cDNA. Subsequently, the qRT-PCR was conducted on an ABI 7500 instrument with an optimized thermal cycling protocol (Table 2). All cycle threshold (Ct) values were standardized, and then, utilizing Microsoft Excel, the normalized values were computed through the application of the 2-ΔΔCT method. Statistical comparisons of means were performed using the Mann–Whitney U test. A significance threshold of P-value<0.05 was adopted for statistical significance.
Table 1. Primers employed for assessing gene expression via quantitative real-time PCR
| Gene Name | Sequence | Product size | Reference |
| MDMX | 5΄-GCCTGCCTTGGTGGTT-3΄ 5΄-CCTAACTGCTCTGATACTGACTC-3΄ |
160 bp | (20) |
| MDM2 | 5΄-AACCACCTCACAGATTCCA-3΄ 5΄-GCACCAACAGACTTTAATAACTTC-3΄ |
87 bp | (21) |
| MYC | 5ʹ-TCACACCCTTCTCCCTT-3ʹ 5ʹ-CGCTCCACATACAGTCC-3ʹ |
180 bp | (22) |
| BIRC5 | 5ʹ-AGTTGGAGTGGAGTCTGG-3ʹ 5ʹ-CTTGCTGGTCTCTTCTGG-3ʹ |
144 bp | (22) |
| EBNA1 | 5ʹ-GGGTGGTTTGGAAAGCATCG-3ʹ 5ʹ-CTTACTACCTCCATATACGAACACA-3ʹ |
156 bp | (22) |
| Beta-actin | 5ʹ-GCCTTTGCCGATCCGC-3ʹ 5ʹ-GCCGTAGCCGTTGTCG-3ʹ |
90 bp | (21) |
Table 2. Thermal cycling protocol for quantitative real-time PCR reaction
| Steps | Time | Cycle | Temperature |
| Initial denaturation | 15 minutes | 1X | 95°C |
| Denaturation | 15 seconds | 40X | 95°C |
| Annealing/Extension | 1 minute | 62°C |
Expression Profiling of Selected Genes Following EBNA1 Transfection
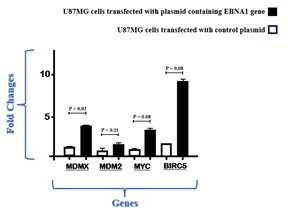
The study assessed the expression of the MDMX gene in U87MG cells that were transfected with EBNA1 in comparison to control cells containing a mock plasmid, as shown in Figure 1. Analysis of real-time PCR data indicated that the presence of EBV-EBNA1 led to an approximately threefold increase in the expression of the MDMX gene (P=0.02). In Figure 1, we also present a comparison of MDM2, MYC, and BIRC5 gene expression levels in cells transfected with EBNA1 and those transfected with a control plasmid. Notably, U87MG cells containing EBNA1 exhibited a higher expression of these three genes compared to cells with the control plasmid.
Figure 1. Alterations in the expression of MDMX, MDM2, MYC, and BIRC5 detected through real-time PCR analysis
Today, it is abundantly clear that viral infections contribute to the development of cancer (23). EBV is among carcinogenic viruses whose role in causing epithelial cancers (such as nasopharyngeal carcinoma and gastric cancer( as well as lymphoid cancers (like Burkitt's lymphoma, Hodgkin's lymphoma, etc.) has been fully proven (24, 25). Although there is a small probability, EBV infection can be seen in the central nervous system (CNS), especially in immunocompromised people (7). In a study by Gaffari et al., it was revealed that the EBV genome was detected in 21.4% of samples from GBM patients but not in control brain tissue specimens. Among these positive cases, 66% were identified as having EBV type 1, 11% as EBV type 2, and 22% as dual positive for both EBV types 1 and 2 (26). Additionally, Fonseca et al. conducted a study using conventional PCR to analyze 75 frozen glioma samples, reporting an incidence of EBV DNA at 14.7% in WHO grade III and WHO grade IV glioma samples (27).
EBV contributes to carcinogenesis through a range of its distinct proteins, and one of these key proteins is EBNA1, which is expressed across all EBV latency types (12). Although EBV-EBNA1 is not yet classified as a direct oncogene, its multifaceted roles have a significant impact. This includes interactions with various cellular proteins, leading to alterations in their functions (28, 29), binding to diverse RNAs (30), and even binding to specific DNA sequences within the host cell's genome. These combined actions make EBNA1 a potent driver of cellular oncogenesis (28, 31). Numerous investigations have demonstrated that this protein can alter the expression of cellular genes by interacting with their promoters (32), and if these affected genes were cellular oncogene types, the host's condition would be highly critical. In our prior research endeavor, we demonstrated that EBNA1 can bind to the promoters of other viral genes and influence their expression (22). Consequently, this highlights that EBV, beyond its mechanisms associated with virulence, possesses the ability to entirely reshape the dynamics of other viral infections within the host, contributing to a heightened exacerbation of the patient's condition.
Our present study revealed that in U87MG cells transfected with an EBNA1-containing plasmid, there was a notable increase in the mRNA expression of MDMX and MDM2 cellular genes when compared to the control cell group. MDMX and MDM2 belong to the MDM family, which is composed of these two proteins and their derivatives (33). MDMX and MDM2 play critical roles in the negative regulation of the p53 tumor suppressor, which is required for synchronized malignancy suppression and maintaining cells (16). Numerous investigations have shown that MDMX and MDM2 were overexpressed in a variety of human tumors, including retinoblastoma, breast cancer, lung cancer, colon cancer, and glioblastoma (34-36). It has been shown that approximately 80% of gliomas have a p53 pathway malfunction, and 40% of gliomas show a p53 mutation or deletion (37), which may be related to MDMX and MDM2 overexpression. Based on the comprehensive assessment which was conducted by Van Meir et al. it was shown that changes in the p53 pathway are important in the development of GBM. Surprisingly, genomic analysis of human GBM genes and their critical pathways revealed that p53 signaling was changed in 87% of GBM cases (38). As part of a study conducted by the American Association for Cancer Research (AACR), it was revealed that MDMX underwent alterations in 5.26% of GBM patients (39). Additionally, a separate investigation led by Riemenschneider and colleagues identified a 5- to 25-fold overexpression of MDMX in 2.4% of 208 cases of glioma (40). In another investigation by Arjona et al., MDMX was shown to be overexpressed in 27% of 86 GBM samples. Moreover, this study revealed a noteworthy increase in the abundance of MDMX in low-grade astrocytic tumors, indicating that this phenomenon might signify an initial event in the progression of carcinogenesis (41). MDMX does not possess E3 ligase activity, and as a result, one of its methods for reducing P53 levels involves forming a heterodimer with MDM2 to activate MDM2's ubiquitination function. This event is commonly referred to as the p53-MDM2/MDMX loop, in which both MDM2 and MDMX jointly restrain the tumor suppressor function of p53 (42). As mentioned, MDM2 plays an imperative role in down-regulating p53 activity via ubiquitin-dependent degradation (42). Additionally, several studies revealed that the p53-ARF-MDM2 pathway was dysregulated in a substantial majority of GBM patients and GBM cell lines (43, 44). In this regard and in agreement with us, Werner et al. showed that MDM2 was overexpressed in 14% of GBM cases (38). Moreover, according to Reifenberger et al., 8–10% of GBM had MDM2 overexpression (45).
Our research findings revealed a notable threefold increase in the expression of the MYC gene in U87MG cells that were transfected with the EBNA1 plasmid when compared to cells transfected with the control plasmid. The great majority of malignancies have unregulated activity of this cellular oncogene, which is controlled as a downstream effector by cell metabolism (46). It has been shown that the MYC protein plays a pivotal role in the development and progress of GBM (47). MYC overexpression has been reported to correlate with a higher grade of malignancy in glioma (48, 49). MYC is in charge of EGFR overexpression at the transcriptional level (50) as well as the expression or transcriptional suppression of some miRs involved in glioma chemoresistance (51, 52). Some MYC tumorigenic effects in GBM are exerted through the molecular partners belonging to its protein network (53, 54). Moreover, MYC is one of the master genes controlling the stem cell characteristics of cancer-initiating glioblastoma stem cells (GSCs) (55). Furthermore, our prior research showcased a substantial threefold augmentation in the expression of the MYC gene in HeLa cells following EBNA1 transfection (22). Consequently, given MYC's multifaceted proto-oncogenic nature, the heightened expression of MYC induced by EBNA1 in astrocyte cells infected with EBV could potentially expedite the onset and advancement of GBM.
Additionally, we identified a great elevation in the transcript level of the BIRC5 gene in U87MG cells transfected with the EBNA1-containing plasmid when contrasted with control cells, although this change was not significant. BIRC5 gene product (also named survivin) is missing in healthy differentiated tissues but is highly expressed in the majority of tumors (56). Survivin controls the progression of the cell cycle, prevents apoptosis, and causes instabilities in chromosomes (57, 58). This protein suppresses cell death by interfering with caspases, which is why its expression has been shown to rise in many cancers (59). Accumulating evidence has revealed that there is a linear relationship between survivin gene transcript level and malignant phenotype in glioma (60). According to Tong et al., the expression of survivin is correlated with a poor prognosis among GBM patients (56). In accordance with our research, a study conducted by Lu and colleagues reported that the viral protein EBNA1 induces the generation of survivin in Burkitt's lymphoma. This induction occurs through the formation of a complex and its attachment to the survivin promoter (61). Additionally, our previous investigation demonstrated a significant enhancement of BIRC5 expression by EBNA1 in a cervical cell line (22).
While our study has successfully identified several differentially expressed mRNAs when comparing EBNA1-transfected cells with the control group, it's important to acknowledge the existence of certain limitations. One critical aspect is the necessity to validate these findings at the protein level using alternative techniques, such as western blotting, to enhance the robustness and reliability of the results. This step should be considered in future investigations to overcome this particular limitation. Additionally, the study was constrained by the availability of only the U87MG cell line for our research, which limits the extent of comparative analysis. To address this constraint and enhance the comprehensiveness of our understanding, we recommend the design of further studies involving a broader range of glioblastoma astrocytoma cell lines or even clinical samples. Such studies could encompass an evaluation of various cellular and viral genes at both the mRNA and protein levels. This approach has the potential to yield novel insights into the intricate interplay of EBV in the development of various brain conditions while shedding light on the multifaceted biological roles of EBNA1 in the context of carcinogenesis. These endeavors would undoubtedly contribute to a more comprehensive understanding of the molecular mechanisms underpinning the influence of EBV on brain health and disease.
The findings from our study provide compelling evidence that the presence of EBNA1 can elevate the expression levels of a range of critical cellular genes, namely MDMX, MDM2, MYC, and BIRC5, within U87MG cells. This observation suggests that EBV infection, facilitated by the EBNA1 protein, can exert a substantial influence on the pathophysiology of GBM. This influence extends to the potential exacerbation of the disease's conditions, thereby underscoring the multifaceted role of EBV in the context of GBM development and progression.
We sincerely thank all colleagues who helped us in carrying out and advancing this study.
This study was conducted in compliance with ethical guideline (IR.SUMS.REC.1401.003) and does not involve any research with human participants or animals.
Conflicts of Interest
No conflicts of interest are disclosed by the authors.
A.H.A., J.S., and A.G.L. designed and administrated the project. A.H.A. wrote the manuscript. S.M.A.H. and H.N. edited the manuscript. A.H.A. and Z.Z.K. performed the experiment. A.H.A. and S.M.A.H. analyzed the data. All authors read and approved the final version of the manuscript.
This work was not supported by any funding.
Received: 2023/12/5 | Accepted: 2024/02/16 | ePublished: 2024/03/18
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |