BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2210-en.html
2- Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
3- Department of Biostatistics and Epidemiology, School of Health and Nutrition, Lorestan University of Medical Sciences, Khorramabad, Iran
4- Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran ,
Hepatitis delta virus (HDV) is a defective RNA virus and requires hepatitis B virus (HBV) surface antigen (HBsAg) for replication. Therefore, infection with HBV is essential for infection with HDV (1). Hepatitis delta virus was discovered in 1977 by Rizzetto in patients with HBV infection. Hepatitis delta antigen (HDAg) was first identified in the hepatocytes of patients with chronic hepatitis B and was distinguished from other HBV antigens such as HBsAg, HBcAg, and HBeAg (2). The HDV genome is a single-stranded negative sense and covalently closed circular RNA with 1.7kb in length (3).
Approximately 250 million people worldwide are infected with HBV, with around 60 million of them also suffering from HDV (4-6). Hepatitis delta infection is prevalent globally, but its occurrence varies in different regions. For instance, the prevalence of HDV in asymptomatic HBsAg-positive carriers is 28.6% in Afghanistan, 16.8% in Somalia, 1.56% in Yemen, 0.9% in Lebanon, and 4.94% in Iran (7). Studies showed different results of anti-HDV among Iranian HBV-positive patients. The prevalence of anti-HDV was reported at 1.2% in Birjand (8), 1.7% in Kermanshah (9), 2.1% in Qom (10), 5% in Hormozgan (11), 5.8% in Mashhad (12), 7.7% in Tehran (13), 17% in Zahedan (14), and 21.8% in Razavi Khorasan province (15).
The prevalence of HDV infection is lower in asymptomatic HBsAg-positive carriers compared to patients with chronic, cirrhotic, and HCC (7). However, the clinical course of HBV/HDV co-infection is more severe, with a three-fold higher risk of HCC and increased mortality compared to patients with HBV alone (16).
All patients with fulminant hepatitis or those with a chronic liver disease caused by HBV should be assessed for HDV infection if their clinical symptoms suddenly change without justifiable reason. Serological methods such as ELISA can be used to diagnose the disease by testing for HDAg and anti-HDV assays. Additionally, molecular methods such as RT-PCR can be used to detect HDV RNA.
The present study is the first in Khorramabad city (western Iran) to estimate the prevalence of HDV among HBsAg-positive patients using serological and molecular methods. This is crucial for providing appropriate and timely treatment to these patients, as accurate statistical data on this specific group is lacking.
Study Population
This study included 200 individuals who tested positive for HBsAg and had a medical record. These individuals were referred to Shahid Rahimi and Shohada Ashayer teaching hospitals, as well as medical diagnostic laboratories in Khorramabad in 2019. Prior to the study, informed consent was obtained from all participants, following ethical guidelines (Ethic code: IR.LUMS.REC.1398.104). 5mL of venous blood samples were collected from each participant in EDTA-free tubes. The blood samples were then separated into serum and used for ELISA and RT-PCR tests to detect HDV infection.
Serological Testing
We performed an ELISA test using an anti-HDV antibody detection kit (Dia. Pro, Italy) according to the kit protocol for analyzing sera from HBsAg-positive individuals. All samples were tested in duplicate. Samples with values above 1.1 were considered positive, while samples below 0.9 were considered negative. Samples falling within the range of 0.9-1.1 were considered borderline.
Molecular Testing
In order to verify the presence of antibodies against HDV in the samples, the RNA was extracted using the High Pure Viral RNA Genome Extraction Kit (Roche, Germany) following the kit protocol. Subsequently, cDNA synthesis was performed using RT enzyme (Thermo Fisher Scientific, Lithuania) and Random Hexamer primer. Specific primers (17) were then used to amplify a 430nt fragment of the HDV delta gene. Briefly, a reaction mixture of 25μL was prepared for the experiment. The mixture consisted of 12.5 µL of PCR Master Mix (Amplicon, Denmark), 1 µL of 10 pmol of each primer (Table 1), 2 µL of cDNA, and 8.5 µL of distilled water. The thermal cycling conditions involved one cycle at 94°C for 5 minutes, followed by 35 cycles of three steps: 94°C for 30 seconds, 58°C for 35 seconds, and 72°C for 30 seconds. The final cycle was at 72°C for 5 minutes, with an infinite hold at 4°C. To check for the presence of HDV in the samples, the RT-PCR products were electrophoresed on a 1.5% agarose gel using a DNA-safe stain and visualized using a gel documentation system.
Table 1. Specific primer sequences for amplification of HDV by RT-PCR tests
| Primers | Primers sequences |
| HDV forward primer | 5- CATGCCGACCCGAAGAGGAAAG -3 |
| HDV reverse primer | 5- GAAGGAAGGCCCTCGAGAACAAGA -3 |
The demographic data was analyzed using SPSS v26 software (IBM, Chicago, IL., USA). To identify differences between groups, the Chi-square test was used. A P-value ≤0.05 was considered statistically significant for all tests and data analyses.
Out of the total participants, there were 119 (59.5%) men and 81 (40.5%) women. The age of the patients varied from 5 to 89 years, with an average age of 49.66 years. Among them, 44 (22%) individuals were single while 156 (78%) were married. Of the surveyed population, 26 (13%) were drug addicted and 174 (87%) were non-drug addicted. 186 (93%) people resided in urban areas while 14 (7%) lived in rural areas (Table 2).
Table 2. The study population includes gender, married status, drug addiction, and residence
| n(%) | ||
Gender |
Male | 119(59.5) |
| Female | 81(40.5) | |
Married status |
Single | 44(22) |
| Married | 156(78) | |
Drug Addiction |
Yes | 26(13) |
| No | 174(87) | |
Residence |
Urban | 186(93) |
| Rural | 14(7) | |
| Total | 200(100) | |
According to the ELISA test results, 37 (18.5%) out of the HBsAg-positive individuals were found to be infected with HDV, while 10 (5%) patients had borderline results for the virus (Table 3).
Table 3. The HBV and HDV viral markers results.
| Test | Positive n(%) | Negative n(%) | Borderline n(%) |
| HBsAg | 200(100) | 0(0) | 0(0) |
| HDV-Ab | 37(18.5) | 153(76.5) | 10(5) |
| HDV-RNA | 42(21) | 158(79) | 0(0) |
Among the 37 HDV-positive patients, 20 (54.1%) were men and 17 (45.9%) were women. Additionally, 5 (13.5%) of the HDV-positive cases were single, while 32 (86.5%) were married. Furthermore, 7 (18.9%) of the HDV-positive cases were drug-addicted, while 30 (81.1%) were not. All ELISA-positive cases resided in urban areas. However, serological tests did not reveal any statistically significant differences between HDV infection and factors such as gender, marital status, drug addiction, or place of residence (Table 4).
Table 4. The HDV ELISA test results
| Risk factors | HDV-Ab Positive n(%) | HDV-Ab Negative n(%) | HDV-Ab Borderline n(%) | P value | |
Gender |
Male | 20(54.1) | 93(60.8) | 6(60) | 0.755 |
| Female | 17(45.9) | 60(39.2) | 4(40) | ||
Married status |
Single | 5(13.5) | 37(24.2) | 2(20) | 0.368 |
| Married | 32(86.5) | 116(75.8) | 8(80) | ||
Addiction |
Yes | 7(18.9) | 13(8.5) | 0(0) | 0.092 |
| No | 30(81.1) | 140(91.5) | 10(100) | ||
Residence |
Urban | 37(100) | 141(92.2) | 8(80) | 0.062 |
| Rural | 0(0) | 12(7.8) | 2(20) | ||
In this study, people with a positive and borderline diagnosis of HDV infection by ELISA were also evaluated to confirm the incidence of HDV by molecular methods. All 37 positive individuals detected using the ELISA as well as 5 of 10 borderline individuals by serological testing (42 of 200 (21%)) were found to be positive by molecular RT-PCR testing (Figure 1).
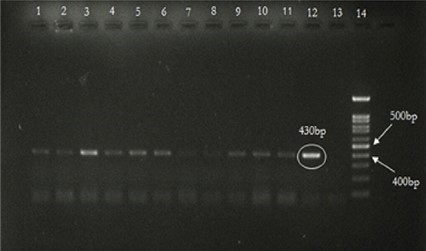
Figure 1. Result of HDV amplification by RT-PCR test. Lanes 1 to 12 show the results of the HDV amplification by RT-PCR test on samples that were positive in the ELISA test. Lane 13 contains negative control. Lane 14 is the 100bp DNA Marker.
RT-PCR test results showed that 42 people in our study were positive for HDV, including 23(54.8%) male patients and 19 (45.2%) female patients. Among the 42 HDV-positive patients, 5 (11.9%) were single and 37(88.1%) were married. Seven (16.7 %) patients were drug addicted and 35 (83.3%) patients were non-drug addicted. All RT-PCR-positive cases were from urban areas. The frequency of HDV-positive cases concerning molecular RT-PCR testing was statistically different between cases living in urban and rural areas (P=0.032) (Table 5).
Table 5. The HDV RT-PCR test results
| Risk factors | HDV-RNA Positive n(%) | HDV-RNA Negative n(%) | P value | |
Gender |
Male | 23(54.8) | 96(60.8) | 0.485 |
| Female | 19(45.2) | 62(39.2) | ||
Married status |
Single | 5(11.9) | 39(24.7) | 0.054 |
| Married | 37(88.1) | 119(75.3) | ||
Drug Addiction |
Yes | 7(16.7) | 13(8.2) | 0.096 |
| No | 35(83.3) | 145(91.8) | ||
Residence |
Urban | 42(100) | 144(91.1) | 0.032 |
| Rural | 0(0) | 14(8.9) | ||
Odds ratio analysis was performed to find risk factors for HDV infection. Among the study cases, the risk assessment for HDV infection showed a lower likelihood in males, single cases, and non-drug addicted cases (Table 6).
Table 6. The HDV infection risk estimation among the studied groups
| Study Group | Odds Ratio | 95% Confidence Interval |
| Male/Female | 0.782 | 0.394-1.553 |
| Married/Single | 2.425 | 0.891-6.602 |
| Addicted/Not Addicted | 2.231 | 0.829-6.005 |
Because the hepatitis D virus is a defective virus requiring the hepatitis B surface antigen (HBsAg) to replicate, some patients infected with HBV also become infected with HDV (1). This co-infection generally results in more severe liver disease, such as HCC, compared to those infected with HBV alone (18). Therefore, it is necessary to determine the prevalence of HDV to plan prevention programs. In this study, we investigated the prevalence of HDV infection among HBsAg-positive patients living in Khorramabad city, Iran.
Various studies show a wide prevalence of HDV in Iran and globally. Differences in HDV infection prevalence may be due to differences in age distribution, sample size, geographic location, cultural differences, health status, and study time. In this study, the incidence of HDV infection in HBsAg-positive patients according to serological and molecular analysis was 18.5% and 21%, respectively. Studies have shown that the prevalence of HDV is highly variable in different regions of Iran, ranging from 0.0% to 21.8% in HBsAg-positive individuals (19). In a study conducted by Froutan et al. among HBsAg-positive patients in Tehran, 206 patients were tested in 2005 and 2006. Serological markers were measured by ELISA. HDV infection was positive in 26 (12.6%) patients (20). In another study conducted by Tahaei at Taleghani Hospital Tehran in 2014, anti-HDV antibodies were detected in 39(7.7%) of 509 HBV chronic patients. In their study, age was significantly associated with HDV seropositivity (13). In a study conducted in Shiraz by Motamedifar in 2012, 35 (19.7%) of 178 patients co-infected with HBV and HIV tested positive for anti-HDV antibodies. They found that HDV infection was not associated with age, marital status, unsafe sex, or injection drug abuse (21). In another study, by Attaran et al. in Iran, 854 asymptomatic HBsAg-positive blood donors were tested in 2014. The presence of anti-HDV antibodies was detected using an ELISA test and subsequent RT-PCR. In this study, 18 (2.1%) of 854 samples were anti-HDV positive and 0.6% were HDV RNA positive (22). In a study by Das et al. in Pakistan, 23 (31.5%) of 73 HBsAg-positive patients had HDV antibodies. HDV RNA was detected in 15 (75%) 23 positive anti-HDV. They found no significant differences between HDV seropositivity and demographic factors such as age, gender, marital status, and place of residence (23). Another study conducted in Nigeria in 2009 tested 96 HBV-positive patients for anti-HDV antibodies. Anti-HDV was detected in 12 (12.5%) patients (24). The prevalence of anti-HDV antibodies in HBsAg-positive patients in the study by Pouri et al. in East Azerbaijan province was 2.17% (25). In another study by Sharifian in Razavi Khorasan province, the prevalence of anti-HDV antibodies among 87 HBsAg-positive patients was 21.8% (15).
Demographic factors such as gender, marital status, addiction, and place of residence may be associated with HDV infection. However, in this study, there was no statistically significant difference between HDV infection and these factors in serological testing, but a statistically significant difference was observed in the incidence of HDV infection between cases living in urban and rural areas in molecular testing.
The relationship between gender and HDV prevalence in our study contradicts previous reports from Razavi Khorasan province, Tabriz, and Pakistan, which showed that women were less likely to be infected with HDV than men (15, 26, 27). The unequal number of men and women in our study may have affected the results. Therefore, more research is necessary in this field.
The findings of the current study indicate that married individuals had a higher rate of HDV infection compared to single individuals. This higher incidence among married individuals may be attributed to the larger number of cases included in this study (67 married and 19 single cases), which warrants further investigation.
The results of this study revealed a higher prevalence of HDV among individuals with a history of addiction. Consistent with our findings, previous studies conducted in Birjand and Tabriz cities also found a higher incidence of HDV among individuals with a history of IV addiction (28, 29). However, there may be a discrepancy between these studies due to several reasons. Firstly, individuals with a history of addiction are more likely to seek healthcare services, including tests and treatment, at health centers. In contrast, our study focused on cases from hospitals and medical diagnostic laboratories. Additionally, the higher number of non-addict individuals in our study may have influenced the results. Therefore, further research is warranted in this field.
Additionally, the study found that people residing in urban areas had a higher HDV prevalence than the rural population. These results contradict previous studies conducted in Golestan province and Pakistan, where the rural population exhibited a higher infection rate. This discrepancy may be attributed to cultural differences and varying high-risk behaviors among the populations in these respective areas (27, 30).
One limitation of our study was the small sample size, which could have impacted the obtained results. Moreover, differences in factors such as age distribution, sample size, and geographical location may contribute to the variation in results observed across different studies.
The findings revealed a significant difference in the frequency of HDV-positive cases between individuals residing in urban and rural areas. Additionally, the prevalence of HDV was higher among men and married individuals in this study. Notably, the prevalence of HDV infection was high among HBsAg-positive cases in Khorramabad city. Therefore, it is recommended to screen HBsAg-positive patients for HDV antibodies. Conducting such evaluations will aid in ensuring timely diagnosis and appropriate treatment for these individuals.
No to declare.
Ethical Considerations
The study was approved by the Ethics Committee of Biomedical Research at Lorestan University of Medical Sciences (LUMS) (ethic code: IR.LUMS.REC.1398.104).
Authors’ Contribution
MA designed the study. NG and MA collected the clinical data and performed experiments. MB and AA analyzed the data. NG and MA wrote the main manuscript. AA and SK reviewed the manuscript. All authors accepted the final version of this manuscript.
This work was supported by the Vice-Chancellor for Research Affairs of Lorestan University of Medical Sciences (grant number: 1197).
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2023/10/28 | Accepted: 2024/01/17 | ePublished: 2024/03/18
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








