BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2203-en.html


 , Gilda Eslami1
, Gilda Eslami1 

 , Hamideh Emtiazi2
, Hamideh Emtiazi2 

 , Mohammad Reza Mozayan3
, Mohammad Reza Mozayan3 

 , Elmira Zarei4
, Elmira Zarei4 

 , Parisa Bagheri5
, Parisa Bagheri5 

 , Elham Rezaee6
, Elham Rezaee6 


2- Faculty of Pharmacy, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3- Department of General Courses, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4- Department of Hematology and Blood Banking, School of Allied, Medical Sciences, Tehran University of Medical Sciences, Tehran, Iran
5- Department of Hematology and Blood Banking, Paramedical School, Gerash University of Medical Sciences, Gerash, Iran
6- Department of Medical Parasitology and Mycology, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran ,
Leishmaniasis is a parasitic illness carried by the bite of some species of sand flies. Protozoan parasites of the genus leishmania cause it (1). Visceral leishmaniasis, targeting several vital organs like the liver, spleen, and bone marrow, along with cutaneous leishmaniasis (CL), known for causing nodules or lesions primarily on facial skin, are the two most common forms of the disease (2). Cutaneous leishmaniasis consists of two forms: urban and rural, called anthroponotic cutaneous leishmaniasis (ACL) and zoonotic cutaneous leishmaniasis (ZCL), respectively (3).
Visceral Leishmaniasis (VL), also known as Kala Azar, is the most severe form of the disease, which has a mortality rate of almost 100 percent in untreated patients. The third type, Mucocutaneous leishmaniasis (MCL), also known as espundia, can cause significant and disfiguring damage to the mucous membranes in the nasal, oral, and throat areas, potentially even affecting the cartilage. On occasion, the cutaneous version of the disease might progress to a more widespread form called Diffuse Cutaneous Leishmaniasis (DCL) (4, 5). The annual prevalence of leishmaniasis in the world is projected to vary from 700,000 to 1.2 million, according to research issued by the World Health Organization (WHO) in 2019 (6). It is a neglected tropical illness with an estimated 12 million patients worldwide and 1.5-2 million new cases annually, including 1-1.5 million cutaneous and 0.5 million visceral cases (7). Despite mounting indications of a rising threat, no vaccinations are available for human CL, and treatment is difficult, particularly in resource-constrained situations (8). Symptoms of cutaneous leishmaniasis can range from single self-healing skin lesions to severe visceralization or persistent metastatic spread (9). A study conducted by the World Health Organization in 2019 revealed that six countries, namely Afghanistan, Algeria, Brazil, Colombia, Iraq, and Syria, along with the Arab Republic and the Islamic Republic of Iran, accounted for over 95% of new cutaneous leishmaniasis cases in 2017 (6). Leishmaniasis is difficult to treat, and currently, the available drugs for leishmaniasis treatment include pentavalent antimony compounds (melamine antimonite and sodium stibogluconate), Miltefosine, Pentamidine, and Amphotericin B. Despite their effectiveness, they can be highly toxic. Moreover, treatment with these drugs is very expensive for governments, making them inefficient considering that major use would be in isolated areas of developing countries where leishmaniasis is extremely associated with poverty (10, 11). Therefore, it is necessary to develop more effective and safer drugs. Plant-based substances are often regarded as less toxic compared to synthetic counterparts. Medicinal plants are considered a valuable resource for discovering new chemical compounds that may possess therapeutic properties (12). Several natural products such as nigella assa foetida, lignans, neolignans, alkaloids, chalcones, and triterpenoids have demonstrated antileishmanial activity (13, 14). Recent research points to the importance of natural honey in regulating the immune system. Famous for its health benefits, natural honey primarily contains sugars such as fructose, glucose, sucrose, and maltose. It also offers a modest quantity of proteins, amino acids, minerals, trace elements, vitamins, aromatic compounds, and polyphenols. Additionally, honey is recognized for its wide range of pharmacological properties, including its role as an antimicrobial, immune system modulator, probiotic, anti-parasitic, anti-inflammatory agent, and pain reliever (15-18). Studies have confirmed that honey can inhibit the growth of bacteria and is effective against pathogenic bacteria, even those resistant to drugs (19). In their study, Zina et al. examined the effects of honey and sugar on the activities of species with anti-leishmaniasis properties in a cultivation setting. They found that both sugar and honey exhibited anti-leishmaniasis characteristics; however, honey demonstrated a stronger anti-leishmaniasis effect (20). In the present study, we investigated the in vitro evaluation of Yazd honey's effect on Leishmania major [MRHO/IR/75/ER] promastigotes in stationary and logarithmic phases.
2.1. Sample Collection
This research was approved by Yazd University of Medical Sciences with the ethical code IR.SSU.MEDICINE.REC.1394.94, and then multifloral honey obtained by Yazd’s experienced beekeepers in the 2016–2017 harvest season was included in the study. Distilled water was used as the solvent in all experiments.
2.2. Source of Parasites
The L. major strain [MRHO/IR/75/ER] promastigotes were obtained from the medical parasitology department at Tarbiat Modares University's School of Medical Sciences. To maintain the L. major strain (MRHO/IR/75/ER), BALB/c mice were used. Amastigotes were collected from the spleens of mice and then converted into promastigotes using Novy-Mac Neal-Nicolle (NNN) medium. Subsequently, the promastigotes underwent a third passage, gradually transitioning from NNN medium to RPMI1640 medium (supplemented with penicillin [100 U/mL], streptomycin [100 μg/mL], glutamine, and 20% fetal calf serum [FCS]), and were kept at 25°C.
2.3. Parasite Counting
A. Using the slide method, a set number of parasites were moved into screw-capped vials filled with 5 mL of RPMI1640 media. To this mixture, varying amounts of Yazd honey were added at concentrations of 6.25, 12.5, 25, and 50 µg/mL, with each concentration replicated six times. Each experimental sequence also included a control setup. The vials containing the promastigotes were placed in an incubator set at 26°C. After four days, the number of organisms in the cultures was determined. To prepare for counting, a 1:10 dilution was made using saline and a specific stain. For staining the promastigotes, 0.4% Trypan blue was used, which colors dead promastigotes blue but leaves living ones unstained, as described by Jaffe et al. in 1987. A neubar slide chamber was then filled, and the promastigotes were counted within 16 small, square sections. To calculate the total number of organisms per milliliter, the formula used was: N (the number counted) multiplied by 10 (to account for the number in 1 cubic millimeter) and then by 10^3 (to convert to the number per milliliter) and again by 10 (to account for the dilution factor).
A single drop that included the parasites was placed on a microscope slide, along with a drop of the drug solution, and then covered with a cover slip. These slides were then inspected under a microscope to observe the parasites, and the proportion of parasites that took up the stain was recorded. For comparison, normal saline was used as a control in this process.
B. Cell Proliferation ELISA, Brdu (Chemiluminescent) Method
The cell proliferation ELISA was conducted following the guidelines provided by Roche Diagnostics GmbH, Roche Applied Science (Mannheim, Germany, March 2005, Cat. No. 11 669 915 001). In summary, a fixed initial density of the parasites was transferred to screw-capped vials containing 5 ml of liquid medium. Different concentrations of honey (6.25, 12.5, 25, and 50 μg) were added to the medium, and the vials were incubated at 6, 12, 24, 48, 72, and 96 hours. Each concentration was tested six times, with a control included in each run. Finally, the XTT assay was performed and evaluated using the ELISA technique.
Dioxy bromoorydin was introduced and incubated at 37°C for 8 hours.
• The supernatant was subsequently removed.
• A fixator was applied to the permeable membrane.
• Anti-oxibromoorydin conjugated with POD was added and allowed to incubate for 3 hours.
• Chromogen was introduced and incubated.
• Lastly, the process was terminated and the reading was taken at 450 nm.
2.4. Statistical Analysis
The findings were presented as the mean ± SEM. To compare the experimental groups, a one-way ANOVA test was performed using GraphPad Prism 5 software. The significance level was set at a P-value < 0.05.
An inhibitory concentration of 50 (IC50) of Yazd honey and Glucantime against the stationary and logarithmic phases of L.major strain [MRHO/IR/75/ER] promastigotes (PMs) was calculated.
The IC50 of Yazd honey against L.major strain [MRHO/IR/75/ER] PMs for Logarithmic Phase PM and Stationary Phase PM was 28.4 and 31.1 μg/mL, respectively. The IC50 of Glucantime against the same strain for Logarithmic Phase PM and Stationary Phase PM Obtained 334.6 and 339.2 μg/mL, respectively. As expected, the IC50 for the stationary phase on both the composition of Yazd honey and Glucantime in terms of a milligram per milliliter is more than the logarithmic Phase.
The Effectiveness of Yazd Honey on the Viability of leishmania Parasite in Culture
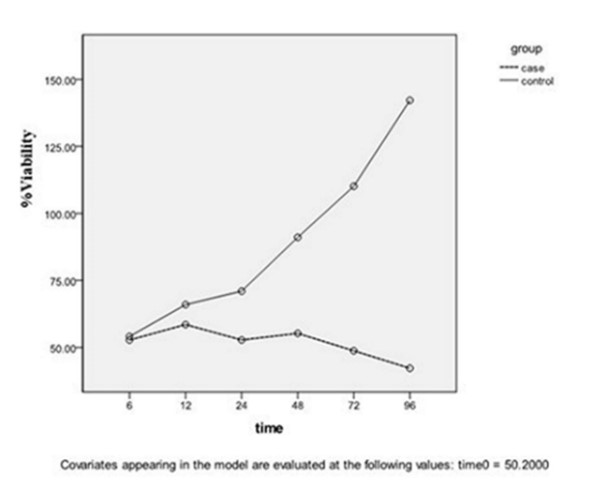
The logarithmic phase: According to Figures 1 and 2, the reduction of live parasite numbers was seen with increasing Yazd honey concentrations (6.25 to 50 µg/ml) and contact duration (6-96 hours) with L. major Iran's strain parasite compared to the control group.
The stationary phase: As shown in Figures 3 and 4, the reduction of live parasite numbers occurred by increasing Yazd honey concentrations (6.25 to 50 µg/mL) and contact duration (6-96 hours) with L. major Iran's strain parasite in comparison with the control group.
As shown in Table 1 and Figure 5, increasing Yazd honey concentrations (6.25 to 50 µg/mL) and contact duration (6-96 hours) reduced the live promastigotes' average numbers in the forms of stationary and logarithmic by the ELISA method
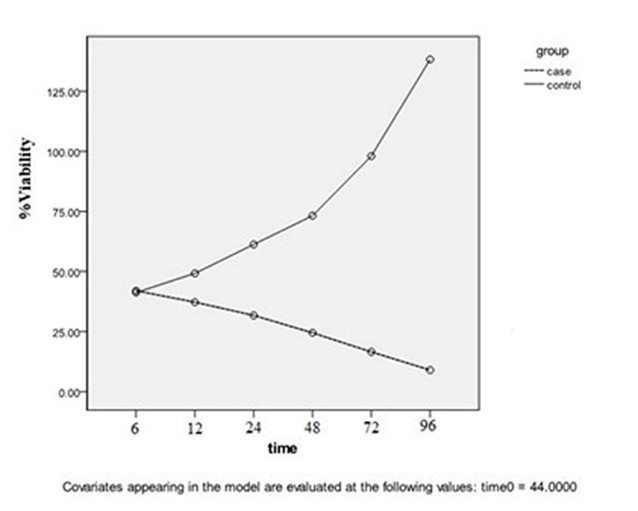
Figure 1. In the logarithmic phase, the concentration of honey was 6.25 µg/mL compared to the control group in different intervals P=0.007.
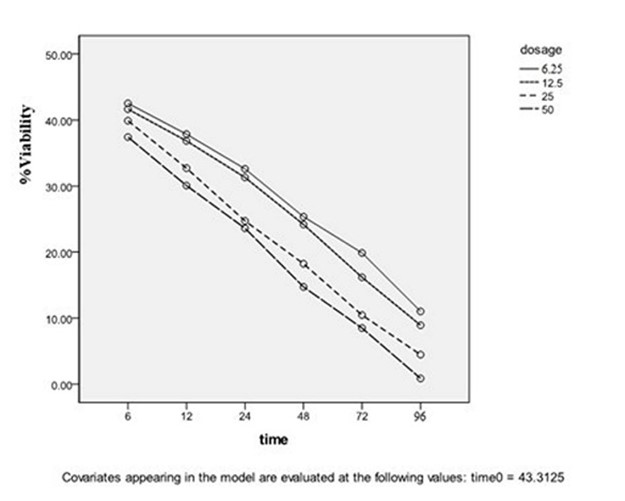
Figure 2. In the logarithmic phase, honey concentrations were 6.25, 12.5, 25, and 50 µg/mL without the control group P=0.002.
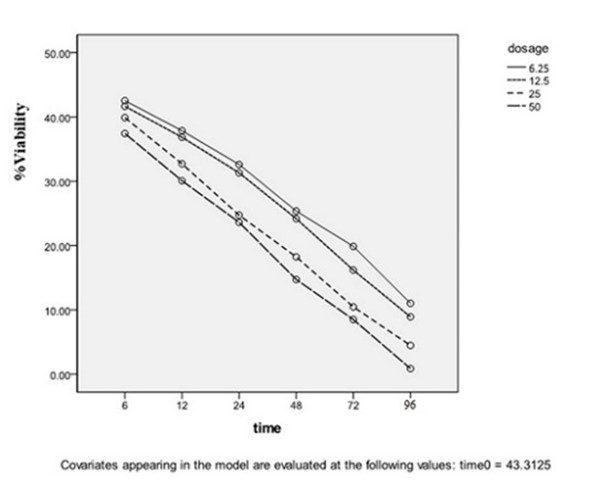
Table 1. The average number of live promastigotes in the stationary and logarithmic forms was evaluated by the ELISA method in concentrations of 6.25, 12.5, 25, and 50 µg/mL Yazd honey, on 6, 12, 24, 48, 72, and 96 hours.
| Stationary Phase Mean±SD |
Logarithmic Phase Mean±SD |
Phases Concentration |
| 175±0.006 | 154±0.005 | Control |
| 151±0.004 | 138±0.003 | 6.25 µg/mL |
| 131±0.003 | 121±0.006 | 12.5 µg/mL |
| 119±0.005 | 112±0.004 | 25 µg/mL |
| 94±0.003 | 86±0.002 | 50 µg/mL |
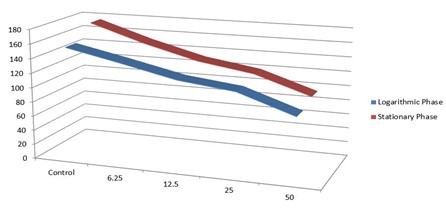
Figure 5. The average number of live promastigotes in the stationary and logarithmic forms evaluated by the ELISA method in concentrations of 6.25, 12.5, 25, and 50 µg/mL Yazd honey, on 6, 12, 24, 48, 72, and 96 hours
Treating leishmaniasis successfully can greatly lessen the social and psychological impact of the disease and help in managing and preventing its spread. The current medications used to treat leishmaniasis are man-made and come with harmful side effects. Conducting pharmaceutical research is key to finding new treatments that are safer and cause less harm. Since ancient times, honey has been traditionally used to treat infections caused by microbes. Recently, Research conducted in labs has shown that honey can combat a range of human pathogens, such as Escherichia coli, Enterobacter aerogenes, Salmonella typhimurium, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), hemolytic streptococci, and vancomycin-resistant Enterococci (VRE) (21).
In their study, AL Zahrani et al. discovered that four types of honey derived from various plants demonstrated effectiveness in combating Staphylococcus aureus Oxa R and S, Pseudomonas aeruginosa, and Candida albicans (22). Research suggests that honey shows potential in combating Candida albicans and Aspergillus niger, as noted by Sheikh and colleagues. Additionally, honey is potent against a variety of fungal threats, including dermatophytes such as Microsporum ferruginous and several strains of Trichophyton, parasites like Allescheria boydii, saprophytic fungi including Mucor mucaralis, and various Aspergillus species (23). Not much research has been carried out on honey’s effectiveness against viruses. However, studies, including one by Ghapanchi and colleagues, have shown that honey at various concentrations can hinder the rubella virus and combat HSV1 (Herpes Simplex Virus 1). These findings suggest honey might be a viable option in traditional medicine for its antiviral properties (24). Research by Critchfield and others observed that flavonoids found in honey, namely chrysin, acacetin, and apigenin, have been shown to block the activation of HIV-1 in dormant infection models. This effect likely works by preventing the virus from replicating its genetic material (25).
In recent years, some studies have been conducted on the effects of honey on the Leishmania parasite in combination with other compounds (extracted from plants, organisms, etc.) or alone. Some of these new studies are mentioned below.
A comprehensive study was conducted by Parvizi et al. in 2020 regarding traditional and herbal treatments for leishmaniasis in Traditional Persian Medicine (TPM). Several oral and topical traditional remedies, including different herbal extractions, honey, and Alum root were mentioned in TPM resources for the treatment of cutaneous leishmaniasis (26).
In 2020, Aksoy et al. investigated the anti-leishmanial effects of various concentrations of pine, flower, and chestnut honey as well as propolis on Leishmania tropica promastigotes in vitro. Results showed that pine honey and propolis were particularly effective in inhibiting parasite growth and suggesting potential as alternative treatments for cutaneous leishmaniasis (27). Although a different species was studied in this research, the outcomes are consistent with those of our study.
In 2022, Gholizadeh et al. conducted a study on the in vitro and in vivo effects of natural honey on Leishmania major. This study found that honey extract, especially at a concentration of 400 μg/mL, has both in vitro lethal effects on Leishmania major parasites and in vivo therapeutic effects on leishmaniasis wounds in BALB/c mice, suggesting further evaluation in human models is warranted (28).
In a 2023 overview, Sinha and colleagues analyzed how manuka honey could be advantageous for controlling infectious diseases. They mentioned that despite the decline in traditional remedy use in modern society, Manuka honey’s antioxidant and antimicrobial properties, especially when used alongside antibiotics, offer a promising approach to enhancing treatment effectiveness against drug-resistant infections including leishmaniasis (29).
In 2023, researchers tested the combined effects of honey and the raw and separated excretions/secretions (ES) from Lucilia sericata larvae against L. major, utilizing the macrophage J774A.1 cell line to gauge anti-leishmanial activity. The results showed that the combination of larval ES fractions and honey acted synergistically against L. major, with significant inhibitory effects on promastigotes and intracellular amastigotes (30). Also, in previous studies, the possible anti-microbial and anti-leishmanial effects of honey have been investigated (20, 31, 32).
The current study’s results demonstrated that Yazd honey effectively inhibited the growth of L. major. The observed parasite counts decreased with higher honey concentrations and longer exposure times compared to the control group.
This study showed that Yazd honey exhibits a greater anti-leishmania activity compared to the control group, with low toxicity and cost, as well as high selectivity and effectiveness in inhibiting the L. major species.
The authors extend their heartfelt thanks to the Vice-Chancellor for Research at Shahid Sadoughi University of Medical Sciences for the financial backing provided for this project. Additionally, they are grateful to all individuals who played a part in bringing this project to fruition.
Ethical Considerations
This research was approved by Yazd University of Medical Sciences with the ethical code IR.SSU.MEDICINE.REC.1394.94, and then multifloral honey obtained by Yazd’s experienced beekeepers in the 2016–2017 harvest season was included in the study. This project is experimental and not epidemiological, so the time factor has no special effect in this project.
Author’s Contribution
A. F. B. and G. E. designed the study. A. F. B. , H. E., E. Z., and P. B. collected the clinical data and performed experiments. A. F. B. and E. R. analyzed the data. A. F. B. and E. R. wrote the main manuscript. M. R. M., A. F. B., and E. R. reviewed the manuscript. Authors accepted the final version of this manuscript.
This research received specific grant based on a project submitted (No. 1202) in the school of medicine, Yazd Shahid Sadoughi University of medical sciences. Yazd, Iran.
Conflicts of Interest
The authors stated no conflict of interest.
Received: 2023/11/27 | Accepted: 2024/02/17 | ePublished: 2024/03/18
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |