BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2200-en.html
2- Medical Technical Institute Al-Mansour, Middle Technical University (MTU), Baghdad, Iraq ,
Human papillomavirus (HPV) remains a significant global health concern as the most prevalent sexually transmitted infection (STI) (1, 2). HPV virus is divided into 2 groups: mucosal and cutaneous, with a range of epithelial hyperplastic lesions. It can also be classified into group A and group B, based on the incidence of malignant lesions. Based on the consistency of their association with cervical and anal cancer, the high-risk strains HPV-16, HPV-18, HPV-31, and HPV-45 are also found in these cancers. HPV virus does not have a polymerase gene so the replication of the viral genome depends on the stimulation of cellular DNA synthesis (3). The widespread prevalence of HPV, particularly its high-risk genotypes, has led to its recognition as a major etiological factor in the development of cervical infections and subsequent cervical cancer (4).
Cervical infections caused by HPV are a leading cause of morbidity and mortality among women worldwide (5). The persistent infection of high-risk HPV genotypes can lead to the progressive transformation of cervical cells, eventually resulting in cervical cancer (1, 6). Although the natural history of HPV infection has been well-characterized, there is still a need to refine our understanding of the immunological mechanisms that underlie disease progression (7, 8). This understanding is crucial for the development of effective diagnostic, therapeutic, and preventive strategies (9, 10).
In recent years, interleukins have emerged as key modulators of the immune response in HPV infections (11). These signaling molecules play pivotal roles in orchestrating various immune processes, including inflammation, immune cell activation, and differentiation (12, 13). However, the specific interactions between interleukins, other immune mediators, and the host immune response in the context of HPV infection remain incompletely understood (14-16).
This study aims to bridge these knowledge gaps by probing into the reactivity of specific interleukins, including the identified isotype of transforming growth factor (TGF), alongside other immune mediators such as interleukin-10 (IL-10) and monocyte chemoattractant protein-1 (MCP-1), in women afflicted by cervical infections caused by HPV. Through the assessment of correlations between these immune mediators and key markers of HPV infection, such as IgM and IgG antibodies against HPV antigens, as well as gene expression profiles, we aspire to shed light on the intricate immunological interplay that underlies HPV-associated cervical infections.
In sum, this research builds upon previous HPV immunity studies by delving into the specific roles of TGF, MCP-1, IL-10, and other immune mediators in HPV-associated cervical infections. By providing a comprehensive view of the immunological landscape, we aspire to contribute to the development of more effective strategies for the diagnosis, treatment, and prevention of HPV-related cervical diseases.
Sample Collection and Participant Enrollment
A total of 50 participants diagnosed with cervical HPV infections and an equal number of healthy controls were prospectively enrolled in this study between January 2nd and August 1st, 2022. Participants were recruited from Al-Elwea Hospital for Delivery and Children and informed written consent was obtained from each participant. Cervical infection diagnoses were established through a combination of cervical cytology (Pap smear) and HPV DNA testing before study inclusion. The histological grade and clinical stage of patients were also documented to provide a comprehensive clinical context.
Detection of HPV Antigens and Antibodies
The presence of IgM and IgG antibodies against HPV antigens was assessed using the enzyme-linked immunosorbent assay (ELISA) technique. Sera obtained from patients were analyzed for the specific detection of HPV antibodies. Furthermore, the prevalence of cytokines, including Transforming Growth Factor (TGF), Monocyte Chemoattractant Protein-1 (MCP-1), Interleukin-10 (IL-10), and Leukotriene B4 (LTB-4), was determined in patient sera using ELISA analysis. The ELISA assay protocol followed the guidelines provided by the manufacturer, and the kits used were from (Cusabio Co Ltd, Germany).
Genetic Analysis
Polymerase chain reaction (PCR) amplification was conducted to analyze the IL-6 gene sequence, resulting in the generation of two fragments, one of 249 base pairs and the other of 431 base pairs (17). This genetic analysis provided insights into specific genetic aspects relevant to the study. DNA for amplification was extracted from cultured cells, and the protocol for tissue collection was strictly adhered to in accordance with established guidelines (18). Standard curves were established through Probe qPCR analysis, employing MmuPV1 gene DNA amplification as a reference. The primer pair (5′-GGTTGCGTCGGAGAACATATAA-3′ & 5′-CTAAAGCTAACCTGCCACATATC-3′) and the probe (5′-FAM-TGCCCTTTCA/ZEN/GTGGGTTGAGGACAG-3′-IBFQ-3′) were utilized for the amplification of the viral E2 region.
Data Analysis
RNA extraction from tissues was carried out using RNA Mini Kits (Applied Biosystems, U.S.A) following the manufacturer's guidelines (19). To ensure the accuracy and reliability of the gene expression analysis, the issue of high Ct values was meticulously addressed by reevaluating the gene expression analysis methods. Data were statistically analyzed using SPSS software version 16.0 (Inc, Chicago, IL, USA). Descriptive statistics, including mean ± SD for continuous variables and numbers with percentages for categorical variables, were employed to present the data. To compare different groups, the Student t-test was utilized. In cases where data related to IL-6 expression were missing, it was clarified that PCR was performed for interleukin 6, with detailed results and interpretation provided (20, 21).
The study adhered to the ethical code number [No. 1216/2022/HS], ensuring the protection of participants' rights, privacy, and well-being while conducting the research.
The demographic analysis of the studied group revealed that there were no significant differences in the mean age between the Papilloma patients (35.02±10.73) and the healthy control group (40.50±13.42) (P=0.2). Furthermore, there were no significant differences among different age groups (20-79 years) in both Papilloma and control groups (P=0.1) (Table 1).
A comprehensive assessment was conducted on a total of 100 participants, including 50 cervical infection samples from Papilloma patients and 50 samples from healthy controls. Significantly higher levels of IgM and IgG antibodies were evident in the patient group in contrast to the healthy controls. The mean±SD of IgM in patients was (88.94±26.04) compared to (16.69±5.84) in controls (P=0.001). Similarly, the mean±SD of IgG in patients was (59.28±44.42) while it was (15.76±5.75) in the control group (P>0.001) (Table 2).
Table 1. Demographical Analysis of the studied group (N=100)
| Parameters | Papilloma group (n=50) |
Healthy Control (n=50) |
P-value | |
| Age (M±SD) | 35.02±10.73 | 40.50±13.42 | 0.2 (N.S) | |
| Age (Years) | (20-29) | 18 (60.0%) | 0.1 (N.S) | 0.1 (N.S) |
| (30-39) | 16 (57.1%) | 12 (42.9%) | ||
| (40-49) | 16 (57.1%) | 8 (50.0%) | ||
| (50-59) | 8 (50.0%) | 15 (71.4%) | ||
| (60-69) | 6 (28.6%) | 2 (66.7%) | ||
| (70-79) | 1 (33.3%) | 1 (50.0%) | ||
Table 2. Comparison of IgG and IgM Levels Between Cases (N=50) and Controls (N=50)
| Type of Igs | Study Groups | Mean±SD | Max | Min | P-value |
| Pap- IgM | Case | 88.94±26.04 | 123.00 | 12.00 | 0.001 (H.S) |
| Control | 16.69±5.84 | 28.00 | 2.00 | ||
| Pap-IgG | Case | 59.28±44.42 | 135.03 | 1.90 | >0.001(H.S) |
| Control | 15.76±5.75 | 31.00 | 2.40 |
Elevated levels of TGF, MCP-1, IL-10, and LTB-4 were observed in the patient group in comparison to the control group. The specific isotype investigated in this study, TGF-β, displayed a mean±SD of (107.43±16.90) in patients compared to (19.52±10.43) in controls (P=0.001). Similarly, the mean±SD of MCP-1 in patients was (64.74±47.50) while it was (11.30±1.43) in the control group (P=0.001). Additionally, the mean±SD of IL-10 in the patient group was (16.96±6.77) compared to (4.14±1.82) in controls (P=0.001). Finally, the mean±SD of LTB-4 in patients was (112.88±36.77) compared to (17.98±8.95) in the control group (P>0.001) (Table 3).
Table 3. Comparison of TGF, MCP-1, IL-10, and LTB-4 Levels Between Cases (N=50) and Controls (N=50)
| Parameters | Study Groups | Mean±SD | Max | Min | P-value |
| TGF (20) | Case | 107.43±16.90 | 160.00 | 88.11 | 0.001 (H.S) |
| Control | 19.52±10.43 | 56.00 | 2.00 | ||
| MCP-1 (105) | Case | 64.74±47.50 | 250.00 | 20.31 | 0.001 (H.S) |
| Control | 11.30±1.43 | 15.00 | 8.00 | ||
| IL-10 (10) | Case | 16.96±6.77 | 40.00 | 6.00 | 0.001 (H.S) |
| Control | 4.14±1.82 | 9.00 | 2.00 | ||
| LTB-4 (109) | Case | 112.88±36.77 | 190.00 | 13.00 | >0.001(H.S) |
| Control | 17.98±8.95 | 54.00 | 3.00 |
The distribution of IgM and IgG levels among the Papilloma patients indicated that 85.7% of the cases had normal levels of both IgM and IgG, while 14.3% had normal IgM levels but elevated IgG levels. Moreover, 66.7% of the cases showed increased levels of IgM but normal IgG levels, and 33.3% had elevated levels of both IgM and IgG. However, these differences were not statistically significant (P=0.1) (Table 4).
Table 4. Distribution of Pap-IgM and Pap-IgG Levels with Cutoff Point
| Pap- IgM | Pap- IgG | Total | p-value | ||
| 1.56-100 | >100 | ||||
| 1.56-100 | N | 30 | 5 | 35 | 0.1 (N.S) |
| % | 85.7% | 14.3% | 100.0% | ||
| >100 | N | 10 | 5 | 15 | |
| % | 66.7% | 33.3% | 100.0% | ||
| Total | N | 40 | 10 | 50 | |
| % | 80.0% | 20.0% | 100.0% | ||
Correlation analysis showed a positive correlation between IgM and IgG levels with TGF, MCP-1, IL-10, and LTB-4 levels (r=.896**, .678**, .692**, .894** respectively) (P=0.001). Additionally, IgG levels were positively correlated with TGF, MCP-1, IL-10, and LTB-4 levels (r=.611**, .528**, .546**, .570**, .618** respectively) (P=0.001) (Table 5).
Table 5. Correlation between IgM and IgG Levels with TGF, MCP-1, IL-10, and LTB-4 Levels
| Parameters | Pap-IgM | Pap- IgG | TGF | MCP-1 | IL-10 | LTB-4 | |
| Pap-IgM | Pearson Correlation | 1 | 0.611** | 0.896** | 0.678** | 0.692** | 0.894** |
| P-value | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| N | 100 | 100 | 100 | 100 | 100 | 100 | |
| Pap- IgG | Pearson Correlation | 0.611** | 1 | 0.528** | 0.546** | 0.570** | 0.618** |
| P-value | 0.000 | - | 0.000 | 0.000 | 0.000 | 0.000 | |
| N | 100 | 100 | 100 | 100 | 100 | 100 | |
**. Correlation is significant at the 0.01 level (2-tailed).
Finally, the HR-HPV gene expression was examined in women infected with Papillomavirus and compared to the control group using RT-PCR. The results indicated a high Ct value in both patient and control groups, suggesting low template concentrations precluding gene expression analysis (Figure 1).
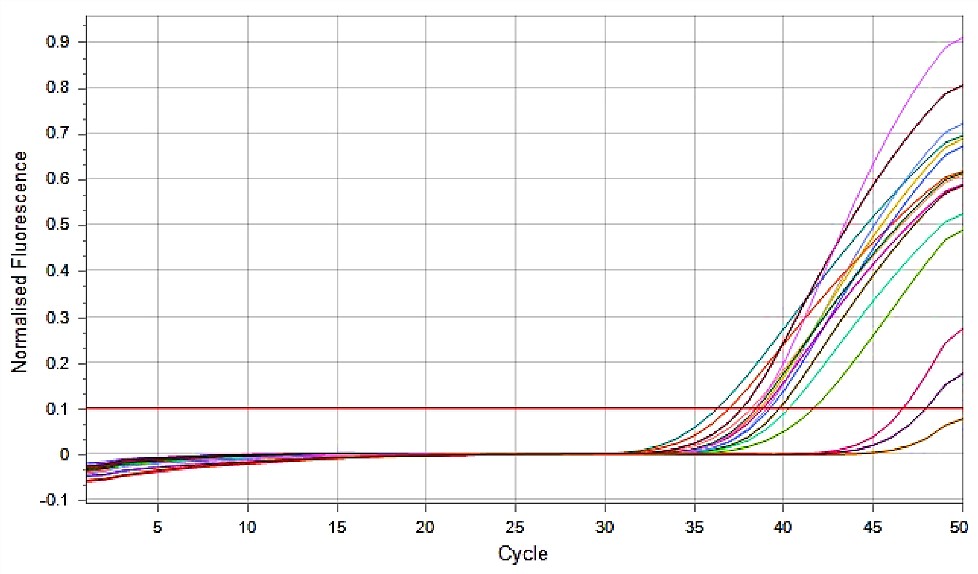
Figure 1. Expression of HR-HPV Gene of Papilloma Virus in Women with Cervicitis Using Real-time PCR (18)
Human papillomavirus (HPV) infection is the most common sexually transmitted infection worldwide, and its association with cervical cancer and other malignancies is well-established (22). In this study, we aimed to investigate the immune response and inflammatory processes associated with HPV infections by analyzing the levels of IgM, IgG, TGF-B1, MCP-1, IL-10, and LTB-4 in Papilloma patients compared to healthy controls.
Our results revealed a significant increase in IgM levels among Papilloma patients, suggesting a robust humoral immune response to the viral infection. This finding is in line with previous studies reporting elevated antibody titers in HPV-infected individuals compared to controls (23). The presence of higher IgM levels may indicate ongoing viral replication and active infection, supporting the potential clinical utility of IgM as a marker for active HPV infections.
Furthermore, the elevated levels of TGF-B1, MCP-1, IL-10, and LTB-4 observed in the patient group indicate the involvement of inflammatory processes in HPV-related diseases. TGF-B1 is a multifunctional cytokine that plays a crucial role in immune regulation and tumor microenvironment modulation. Its increased expression in the patient group is consistent with previous findings implicating TGF-B1 in tumor progression and immune evasion in HPV-related cancers (18).
The significant increase in MCP-1 levels in Papilloma patients may reflect an inflammatory response to the viral infection. MCP-1 is a chemokine that recruits monocytes and macrophages to the site of inflammation and has been associated with various viral infections, including HCV, HAV, and HPV (20). The upregulation of MCP-1 in HPV-infected individuals might contribute to the recruitment of immune cells and subsequent antiviral immune response.
Likewise, the elevated levels of IL-10 observed in the patient group indicate an immune regulatory response to HPV infection. IL-10 is an anti-inflammatory cytokine that plays a critical role in dampening immune responses to prevent excessive inflammation and tissue damage. However, its overexpression can also suppress antiviral immunity and promote viral persistence (21). The increased IL-10 levels in Papilloma patients may reflect an attempt by the immune system to control inflammation caused by HPV infection (24, 25).
Furthermore, our study identified elevated levels of LTB-4 in HPV-infected individuals. LTB-4 is a potent pro-inflammatory mediator that plays a role in the recruitment and activation of leukocytes during inflammatory responses (26). Its upregulation in Papilloma patients suggests a pro-inflammatory environment associated with the viral infection (27).
The positive correlation observed between IgM and IgG levels with TGF-B1, MCP-1, IL-10, and LTB-4 highlights the interconnectedness of the immune and inflammatory responses in HPV infections. This suggests that the host's immune system engages in a complex interplay with the inflammatory pathways to mount an effective antiviral response (28, 29).
While our findings shed light on the immune and inflammatory aspects of HPV infections, several limitations should be acknowledged. Firstly, the nature of the study design limits our ability to establish causal relationships between immune markers and HPV infections. Longitudinal studies with larger sample sizes are warranted to assess the dynamic changes in immune responses during the course of HPV infections and related diseases.
Additionally, our study focused on a specific group of Papilloma patients, and the results may not be fully generalizable to other populations or HPV subtypes. Further research involving diverse populations and various HPV genotypes is necessary to obtain a comprehensive understanding of the immune and inflammatory responses to HPV infections.
In conclusion, our study provides significant insights into the immune and inflammatory alterations associated with HPV infections. Elevated IgM levels indicate active viral replication, while increased TGF-B1, MCP-1, IL-10, and LTB-4 levels suggest immune modulation and inflammation, potentially serving as diagnostic and prognostic indicators. The positive correlation between IgM and IgG levels with TGF, MCP-1, IL-10, and LTB-4 highlights the interplay between immune markers and inflammatory mediators in HPV infections. However, the observed high Ct values in HR-HPV gene expression indicate challenges in gene detection due to limited viral replication.
This research was entirely self-funded. The authors express their gratitude to the Middle Technical University and Al-Elwea Hospital for providing access to their facilities for conducting the study.
The study adhered to the ethical code number [No. 1216/2022/HS], ensuring the protection of participants' rights, privacy, and well-being while conducting the research.
None.
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2023/06/17 | Accepted: 2023/09/9 | ePublished: 2023/09/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








