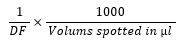BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2168-en.html
2- Department of Biology, College of Science, Mustansiriyah University, Baghdad, Iraq
3- Department of Biology, College of Science, University of Baghdad, Baghdad, Iraq
Antibiotic-resistant infections are widespread worldwide. The escalating resistance of pathogenic bacteria to a wide array of antibiotics has emerged as a significant global concern, primarily attributed to the extensive use of antibiotics in combating various bacterial infections (1, 2). Consequently, the pressing need to develop novel antimicrobial agents produced naturally by bacteria can combat pathogenic organisms with multi-drug resistance (3, 4).
Lactic acid bacteria (LAB), a group of generally recognized as safe microorganisms, have found widespread application in both the food and pharmaceutical industries. One of the notable attributes of LAB is their ability to confer health benefits by demonstrating antagonistic activity against numerous pathogens (5, 6). A pivotal class of metabolites produced by LAB, known as bacteriocins, plays a significant role in their antimicrobial action. Although bacteriocins can be broadly categorized as antimicrobial agents, they diverge from traditional antibiotics in crucial ways (7, 8). Notably, bacteriocins exhibit a restricted spectrum of activity, targeting strains related to their producing species and specifically strains within the same species. Moreover, bacteriocins are ribosomally synthesized antimicrobial peptides or complex proteins produced during the primary phase of bacterial growth, in contrast to antibiotics that are typically secondary metabolites (7, 9).
Bacteriocins have garnered considerable attention from researchers and have been extensively studied due to their potential as alternatives to conventional antibiotics. These bioactive compounds boast several advantages, including physical stability, non-toxicity, and susceptibility to degradation by proteolytic enzymes, particularly those present in the mammalian gastrointestinal tract, rendering them safe for human consumption (10). The production of bacteriocins serves as a critical criterion in the selection of probiotic strains, and the quantity of bacteriocins generated by Lactobacillus is contingent upon the environmental conditions in which these bacteria thrive (11).
Prior research has demonstrated that supernatants of Lactobacillus spp. isolates exhibit varying degrees of inhibitory activity against several pathogenic bacteria, such as Bacillus subtilis, Escherichia coli, Salmonella typhi, Staphylococcus aureus, and Vibrio cholera (12). The emergence of antibiotic resistance has spurred researchers to explore innovative strategies to combat pathogens effectively. As a result, this study aims to investigate the bioactivity of active bacteriocins produced by Lactobacillus spp. isolates and evaluates their antimicrobial effects against different pathogenic bacteria, with the ultimate goal of offering potential alternatives to traditional antibiotics.
Samples Collection and Isolation of Lactobacillus spp:
A total of 140 samples were collected from various sources, including feces, high vaginal wall of healthy women, mouths of healthy infants, breast milk, and commercially available yogurt. The samples were obtained from the Microbiology Unit at Al-Batool Teaching Hospital, from private clinics, and from yogurt stores. For isolation of Lactobacillus spp from clinical samples, the swabs were immersed in 9 ml of De Man, Rogosa, and Sharpe Broth (MRS) broth (Merck, Germany), while for the isolation of Lactobacillus from liquid and solid samples, each tube containing 9 ml of MRS broth was inoculated with 1 ml of liquid sample or 1 g of solid sample and mixed gently to get a uniform sample, separately. All inoculated tubes were incubated at 37°C for 48h under anaerobic conditions, followed by tenfold serial dilution and sub-culture on solid MRS (Merck, Germany) supplemented with 1% calcium carbonate incubated at 37°C in 5% CO2 conditions. After incubation, large white colonies were selected and purified by streaking on MRS agar plates (13). Purified isolates were stored in glycerol stock at -20°C for subsequent analysis (14). Identification tests were performed based on Gram staining, growth on selective MRS and SL agar, and biochemical tests, including catalase, and oxidase. Also, all isolates were tested for acid production ability on MRS supplemented with calcium carbonate by forming a lysis zone around a colony of tested bacteria (15). Moreover, only the Gram-positive, catalase, and oxidase-negative isolates that could be candidates as Lactobacillus spp.
Molecular identification was achieved by sequencing the 16S ribosomal RNA (16S-rRNA) for producer Lactobacillus isolate. Genomic DNA was extracted using the Mini Kit Promega. PCR amplification was carried out with the primers 27F (AGAGTTTGATCCTGGCTCA) and 1492R (GGTTACCTTGTTACGACTT) (16) were supplied by Macrogen Company (Korea) in a lyophilized form. The PCR products were sequenced following the ruction manuals of the sequencing company (Macrogen Inc. Geumchen, Seoul, South Korea) using an ABI (Applied Biosystem) automated DNA sequencer. The sequencing results were edited, aligned, and analyzed along with the respective sequences in the reference database using BioEdit Sequence Alignment Editor Software Version 7.1. sequenced following the ruction manuals of the sequencing company.
List of Clinical Isolates
Clinical isolates used in the study including Pseudomonas. aeruginosa, klebsiellia.pneumoniae, Acinetobacter.baumannii, Kocuria. Kristinae, E. feacalis and Strep. agalactiae.
Screening of Lactobacillus spp. for Bacteriocin Production
Lactobacillus isolates were subjected to screening to identify the most potent bacteriocin-producing isolate to be used for further experiments in this study:
A. Agar-plug Diffusion Method
After overnight growth in MRS broth, A volume of 100 μL of 1.5×108 CFU/mL (McFarland tube No. 0.5) of fresh Lactobacillus isolates were transferred and spread on MRS agar plates and incubated anaerobically at 37°C for 48 hours. Plugs o about 0.5 cm in diameter were made with a sterile cork borer from each isolate which then placed on plates surface of Mullar-Hinton agar streaked with 100 μL of 1.5×108 CFU/mL of pure overnight growth culture of the indicator bacteria, the plates were incubated at 37˚C for 24 hours. After incubation, the diameter of inhibition zones around each plug was measured (15).
B. Agar-Wells Diffusion Method
Well diffusion assay was used to estimate the production of bacteriocin by isolates as follows: Lactobacillus isolates were inoculated in MRS broth under anaerobic conditions at 37°C for 48 hours. The cultures were centrifuged at 6000 rpm for 15 minutes. The cell-free supernatant (CFS) of each isolate was assayed for the presence of bacteriocin using agar well diffusion assay as follows: A 100 μL of 1.5×108 of indicator bacteria suspension was transferred and spread on Mullar-Hinton agar. Wells cut into the pour plates with 5mm sterile cork borer were filled with 100 μl of the CFS. The plates were kept at room temperature for 2 hours and then incubated at 37°C for 18-24 hours (17, 18). Finally, the inhibition zones formed around the wells were measured in mm, compared with that of the control which contained MRS broth only. The method was done with 2 replicates.
C. Filter Paper Disc Method and Agar-Wells Diffusion Method
Lactobacillus isolates that recoded antimicrobial activity in screening were inoculated in MRS broth and incubated anaerobically at 37°C for 48 hours and then CFS (crude bacteriocin) was obtained by centrifuging at 6000 rpm for 15 minutes. The CFS was neutralized to pH 6.5 with 1N NaOH and then treated with catalase and sterilized by filtration through 0.45μm membranes. 100 μl of 1.5×108 CFU/mL of pure overnight growth culture of indicator bacteria was transferred and spread on Mullar-Hinton agar. In the filter paper disc method; sterile filter paper discs measuring 5 mm diameter, saturated with 100 μL of CFS were placed on the Mullar-Hinton agar, and inhibition zones formed around the paper discs were recorded (17, 18).
To confirm the bacteriocin effect and the inhibitory activity of the neutralized CFS with 1N NaOH and treated with catalase (to neutralize the effect of organic acid and H2O2 activity respectively) were screened by agar wells diffusion method, wells cut into the pour plates with 5mm sterile cork borer were filled with 100 μl of the CFS (18-20). All the plates were left in laboratory temperature for 2 hours and then incubated aerobically at 37°C for 18-24 hours. The inhibition zones formed around wells were measured in mm and recorded. One Lactobacillus isolate was selected as a bacteriocin producer. According to the results of screening, the most efficient isolate of Lactobacillus spp. in the bacteriocin production. And the most sensitive isolates of pathogenic bacteria were selected as indicator and used in the subsequent experiments.
Cell-Free Supernatant Concentration
Supernatants of producer isolate were concentrated twice by incubated at 40°C for two days or by evaporation, the antibacterial activity of concentrated bacteriocin was determined against indicator bacteria (21).
Bacteriocin Activity Assay
The concentrated crude bacteriocin was serially diluted two-fold with physiological saline solution. The dilutions were used to assess the bacteriocin's antagonistic activity against the indicator bacteria using agar-well diffusion assay. The highest dilution producing a zone of inhibition growth was calculated as arbitrary units (AU) using the formula (20):
Statistical analysis
All data were analyzed using SPSS 26(SPSS Inc., Chicago, Ill., USA) and Graph Prism (Ver.8) program. The statistical analyses were performed using the analysis of variance independent (t-test) and Chi-square test. Data were presented as a percentage, mean, and SE. A P-value<0.05 was considered significant.
Isolation and Identification of Lactobacillus spp.
A total of 140 samples were collected from various sources, including the vagina, feces, mouth, breast milk, and yogurt. Only Gram-positive, catalase, and oxidase negative were isolated, from the total number of the samples, only 60 Lactobacillus spp. were isolated, with the highest number of isolates obtained from the vagina (28 isolates, 47%), followed by the mouth (7 isolates, 11.6%), breast milk (3 isolates, 5%), feces (3 isolates, 5%), and yogurt (19 isolates, 31.6%). All isolates were surrounded by clear zones after being cultured on MRS agar containing calcium carbonate as a result of dissolving by the acid produced by the bacteria which was described as high acid-producing ability.
Screening for Bacteriocin Production
All isolates that were confirmed through the identification process were subjected to screening techniques in order to select the most efficient isolate as bacteriocin producer, As well as, the most sensitive bacterial isolates were selected as indicator.
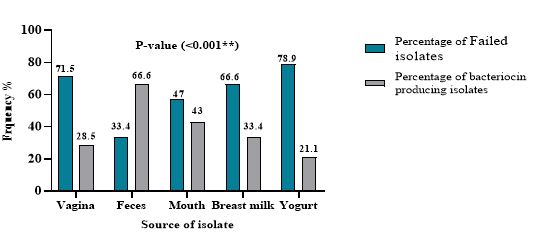
The Lactobacillus isolates were screened for their ability to produce bacteriocins by different methods, the results showed that the agar-plug diffusion method did not appear any antibacterial activity. While by agar well diffusion assay, the number of Lactobacillus spp. showed strong antibacterial activity against Gram-positive bacteria (two isolates of Enterococcus feacalis) and 3 isolates of Gram-negative bacteria (three isolates of P. aeruginosa, one isolate of Acinetobacter baumanni ) with the range of inhibition zone diameters between (7.1 ± 0.14) and (17.75 ± 0.49), also Lactobacillus spp. showed no inhibition zone against other indicator bacteria such as Kocuria. Kristinae and Strep. agalactiae. The highest effective strains against pathogenic bacteria were coded Lh2 DF, La1DV, Lp3DV, Lh1DF, La2 DV, Lp10DV, and Lp14DV (Table 1). There were no significant relationships between the source of Lactobacillus isolation and the production of bacteriocin (Figure 1). Thus, the selection of isolates for screening by filter paper disc method relied on the results of this method.
The size of the inhibitory zone for seven selected isolates against the four indicator bacteria (more sensitive indicator bacteria) by filter paper disc method is shown in (Table 2). However, the strains coded La1DV isolated from the vagina, Lh1DF and Lh2DF isolated from feces showed high inhibitory activity against the indicator bacteria including two isolates of P.aeruginosa and two isolates of Enterococcus feacalis with inhibition zone ranging from (7.16 ± 0.17) to (15.4 ± 0.43).
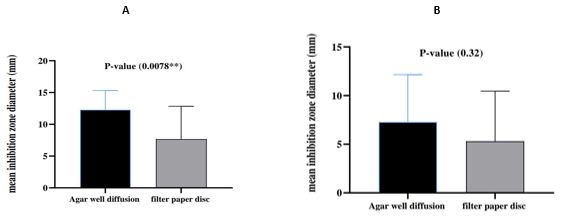
In our study, to confirm the bacteriocin effect neutralized CFS were screened for their inhibition activity by agar well diffusion method. The result obtained with neutral CFS showed that the zone of inhibition became lower compared with the activity of CFS before neutralization by agar well diffusion method (Table 3). Therefore, the result shows that the inhibition activity was not related to lactic acid or H2O2 but might be due to other antimicrobial substances such as bacteriocin. Thus, depending on the results of the screening (Table 3), the most efficient Lactobacillus isolates (Lh2DF) were selected as bacteriocin producers. Also, the most sensitive bacterial isolates including two isolates of Gram-negative bacteria such as P. aeruginosa (A) and P. aeruginosa (B), and two isolates of Gram-positive bacteria such as E. faecalis (A) and E. faecalis (B) were selected as indicator bacteria due to their higher sensitivity toward Lactobacillus isolates. The inhibition zones of L. helveticus DF against P. aeruginosa (A) and P. aeruginosa (B) were (14.1±0.40) and (15.4±0.43), respectively while the inhibition zones against E. faecalis (A) E. faecalis (B) were (10.3±0.27) and (9.9±0.121), respectively. The result of this experiment showed that both the agar well diffusion method and filter paper disc method showed antibacterial activity against more sensitive indicator bacteria (P. aeruginosa (B) and E. faecalis (A)) (Figure 2).
Figure 1. Percentage of bacteriocin producing and non- producing Lactobacillus isolates from different source.
Table 1. Antibacterial activity of Lactobacillus isolates against pathogenic bacteria by agar-wells diffusion method.
| Lactobacillus Isolates | P. aeruginosa (A) | P. aeruginosa (B) | P. aeruginosa (C) | A.baumanni | E. feacalis(A) | E. feacalis(B) |
| Lp3DV | 10.1 ± 0.125 | 11.9 ± 0.20 | 10 ± 0.122 | 9.6 ± 0.142 | 7.78 ± 0.09 | 0 |
| Lp5DV | 8.21 ± 0.20 | 8.2 ± 0.23 | 0 | 0 | 0 | 9.8 ± 0.30 |
| Lp6DB | 8.95 ± 0.08 | 0 | 0 | 0 | 8.3 ± 0.11 | 11.1 ± 0.21 |
| Lp10DV | 10.12 ± 0.2 | 12.5 ± 0.24 | 10.89 ± 0.25 | 8.8 ± 0.13 | 9.33 ± 0.13 | 0 |
| LP13DV | 10.87 ± 0.21 | 7.1 ± 0.14 | 0 | 0 | - | 8.2 ± 0.143 |
| Lp14DV | 8.17 ± 0.172 | 7.9 ± 0.11 | 7.45 ± 0.34 | 10.1 ± 0.123 | 10 ± 0.28 | 0 |
| Lp17DV | 9.4 ± 0.412 | 10.5 ± 0.27 | 0 | 0 | - | 0 |
| La1DV | 13.65 ± 0.40 | 15.9 ± 0.11 | 15.12 ± 0.20 | 10.7 ± 0.244 | 9.31 ± 0.122 | 10 ± 0.271 |
| Lh1DF | 13.32 ± 0.31 | 12.8 ± 0.07 | 10.6 ± 0.16 | 10.9 ± 0.21 | 9.12 ± 0.121 | 0 |
| Lp20DY | 13.6 ± 0.20 | 7.76 ± 0.21 | 0 | 6.8 ± 0.131 | 7.7 ± 0.088 | 0 |
| Lh2 DF | 8.4 ± 0.30 | 17.75 ± 0.49 | 13.67 ± 0.23 | 9.34 ± 0.15 | 12.17 ± 0.31 | 10.85 ± 0.29 |
| La2 DV | 7.3 ± 0.12 | 10.7 ± 0.13 | 8.2 ± 0.12 | 0 | 9.9 ± 0.132 | 10 ± 0.28 |
| Lp22DY | 7.55 ± 0.14 | 9.03 ± 0.145 | 0 | 10.2 ± 0.102 | - | 7.6 ± 0.09 |
| LP30DY | 8.1 ± 0.18 | 9.3 ± 0.11 | 0 | 0 | 9.7 ± 0.129 | 0 |
| LP38DY | 8.01 ± 0.10 | 11.8 ± 0.19 | 0 | 8.4 ± 0.43 | 0 | 0 |
Antibacterial activity of CFS of 15 probiotic bacteria against pathogenic bacteria inhibitory zone diameters expressed in mean±SD.
Table 2. Antibacterial activity of Lactobacillus isolates against pathogenic bacterial isolate by filter paper disc method.
| Lactobacillus no. | P. aeruginosa (A) | P. aeruginosa (B) | E. feacalis(A) | E. feacalis(B) |
| Lp3DV | 10.92 ± 0.30 | 7.55 ± 0.23 | 0 | 0 |
| Lp10DV | 8.76 ± 0.35 | 11.17 ± 0.31 | 8.02 ± 0.09 | 0 |
| Lp14DV | 0 | 7.86 ± 0.25 | 8.86 ± 0.11 | 0 |
| La1DV | 12.68 ± 0.38 | 11.2 ± 0.33 | 10.1 ± 0.251 | 8.1 ± 0.141 |
| Lh1DF | 10 ± 0.26 | 13.08 ± 0.41 | 7.16 ± 0.17 | 0 |
| Lh2 DF | 14.1 ± 0-.40 | 15.4 ± 0.43 | 10.3 ± 0.27 | 9.9 ± 0.121 |
| La2 DV | 7.84 ± 0.24 | 10.1 ± 0.24 | 7.52 ± 0.13 | 10.95 ± 0.29 |
Antibacterial activity of CFS of probiotic bacteria against pathogenic bacteria (inhibitory zone diameters expressed in mean ± SD).
Table 3. Antibacterial activity of neutralized CFS of lactobacillus isolates against pathogenic bacterial isolate by agar well diffusion method.
| Lactobacillus no. | P. aeruginosa (A) | P. aeruginosa (B) | E. feacalis(A) | E. feacalis(B) |
| Lp3DV | 10.92 ± 0.30 | 7.55 ± 0.23 | 0 | 0 |
| Lp10DV | 8.76 ± 0.35 | 11.17 ± 0.31 | 8.02 ± 0.09 | 0 |
| Lp14DV | 0 | 7.86 ± 0.25 | 8.86 ± 0.11 | 0 |
| La1DV | 12.68 ± 0.38 | 11.2 ± 0.33 | 10.1 ± 0.251 | 8.1 ± 0.141 |
| Lh1DF | 10 ± 0.26 | 13.08 ± 0.41 | 7.16 ± 0.17 | 0 |
| Lh2 DF | 14.1 ± 0-.40 | 15.4 ± 0.43 | 10.3 ± 0.27 | 9.9 ± 0.121 |
| La2 DV | 7.84 ± 0.24 | 10.1 ± 0.24 | 7.52 ± 0.13 | 10.95 ± 0.29 |
Antibacterial activity of CFS of probiotic bacteria against pathogenic bacteria (inhibitory zone diameters expressed in mean ± SD).
Figure 2. Compare between Agar well diffusion and Filter paper disc method A: P.aeruginosa B: E. faecalis.
Molecular identification
Lh2DF isolate was subjected to molecular identification using 16S rRNA gene sequencing. The sequencing reactions of the Lh2 DF isolate indicated the exact identity after performing NCBI blasts for these PCR amplicons. The NCBI BLASTn engine also showed 98% sequence similarities between the sequenced sample and Lactobacillus helveticus reference sequences. By comparing the observed nucleic acid sequences of these investigated Lh2 DF isolates with the retrieved nucleic acid sequences (GenBank acc. KX247766.1).
Bacteriocin Activity Assay
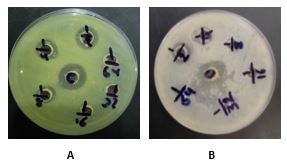
The bacteriocin activity of Lh2DF, identified as Lactobacillus helveticus, was further assessed as the inverse of the last dilution at which growth inhibition was still detectable following the agar well diffusion assay against multidrug-resistant bacteria (Pseudomonas aeruginosa and Enterococcus faecalis). Bacteriocin activity was 40 and 80 AU/mL against E. feacalis(A) and P. aeruginosa (B), respectively (Figure 3).
Figure 3. Bacteriocin activity of CFS from L. helveticus DF concentrated twice. (a) 80 AU/mL against P. aeruginosa(B), (b) 40 AU/mL against E. faecalis(A).
The rise of antibiotic resistance has become a major global concern, therefore the use of probiotics has attracted a lot of interest recently. In this study, we focused on isolating Lactobacillus spp from various sources to achieve suitable strains. In fact Lactobacillus spp dispersed in nature such as dairy, vegetables, and grains, they are also found in specific parts of the body such as the vagina, oral cavity, gastrointestinal and urogenital tract, and play an important role in the exclusion of pathogens (22, 23), the quality of bacteriocin produce from Lactobacillus spp. may well differ according to their source. Antimicrobial activity of Lactobacillus strains against bacterial pathogens emerges to be multi-factorial and attributed to the production of active metabolites such as lactic acid, acetic acid, propionic acid, hydrogen peroxide, and bacteriocins, bacteriocin has a wide range of activity and is generated by both Gram-positive and Gram-negative bacteria (24). The antibacterial mechanisms of bacteriocin can be classified into two categories: Bacteriocin act in sensitive cells by forming pores in the cytoplasmic membrane, and disrupt cell membrane permeability and integrity when bacteriocin exert their antimicrobial effects through intracellular action, they disrupt normal cellular metabolism which causes an efflux of intracellular contents such as potassium, ATP and lactic dehydrogenase resulting in depolarization of the membrane and consequently cell death. The mechanism of action of bacteriocin is complicated each type of bacteriocin can employ diverse antibacterial mechanisms to exert its antibacterial action (25).
The first part of this study was about the morphological, microscopic, and biochemical identification of Lactobacillus spp and its isolation from different sources, then molecular identification was used to confirm biochemical tests (26). According to our findings, gram-positive, rod-shaped bacteria, catalase, and oxidiase negative, belong to the genus Lactobacillus. The second part of this study was about screening Lactobacillus spp. isolates for bacteriocin production, Searching for the best producer isolate to be used in the subsequent step in this study, clinically isolated L. helveticus DF has the potential to produce bacteriocin that can be used as an alternative to antibiotics in the treatment of many nosocomial infection. The well diffusion assay revealed that Lactobacillus spp isolates reproducibly exhibited antibacterial effects against Gram-positive and Gram-negative indicator strains. This method allowed for diffusion of the bacteriocin into the agar medium before indicator strains began to grow (27, 28). In 2022 study by Champiri et al. (29) reported that the well diffusion method had better and more positive results than the disk diffusion method, additionally, we observed that the inhibitory activity of lactobacilli could vary between liquid and solid media, with liquid media demonstrating superior performance due to better diffusion of the bacteriocin substance secreted by Lactobacillus (30). In previous study by Ghorani (31) reported that probiotic metabolites can also be used in medical applications as antiviral, probiotic metabolites are active against various viruses like membrane and non-membrane viruses. The most significant antiviral compounds of probiotic metabolites are hydrogen peroxide, organic acids, and protein molecules. P.aeruginosa main cause of nosocomial infections due to its resistance to the broad spectrum antibiotics, Exposure to antimicrobial drugs for long periods is the most common cause widespread of resistance among Gram-negative bacteria (32, 33).
Moreover, we investigated the impact of concentrated bacteriocin on its antibacterial activity. Consistent with findings by Aziz et al. (34) in lactococcus spp., we observed that the concentration of L. helveticus DF's bacteriocin led to enhanced inhibitory activity against multi-drug resistant bacteria which include Gram-negative bacteria (P. aeruginosa (B)) are highly sensitive in compared to Gram-positive bacteria E. feacalis (A), Bacteriocins are cationic peptides interact with negatively charge outer membrane of Gram-negative bacteria and the outer membrane became leaky, which allow bacteriocin to permeate, leading to cell lysis. Similar results were obtained by Jamalifar (35). Bacteriocin of Lactobacillus isolated from feces showed strong antibacterial activity against Pseudomonas aeruginosa.
This observation highlights the potential for using L. helveticus DF's bacteriocin as an effective antibacterial agent against a broad range of pathogenic microorganisms and underscores its significance as a bio-therapeutic microorganism. Utilizing friendly bacteria like Lactobacillus species as antimicrobial agents offers a promising strategy to address the escalating antibiotic resistance problem (36).
While our study provides valuable insights into the antibacterial potential of L. helveticus DF's bacteriocin, it is essential to acknowledge some limitations. First, the study focused on a limited number of Lactobacillus isolates from specific sources, which may impact the generalizability of the findings. Further investigation with a larger and more diverse sample set would strengthen the study's conclusions. Additionally, we only assessed the in vitro antibacterial activity of the bacteriocin. Future studies should explore its efficacy in vivo and evaluate its safety profile for potential clinical applications.
In the present study, L. helveticus DF emerged as the most efficient isolate for bacteriocin production. L. helveticus DF exhibited antagonistic activity against indicator bacteria including E. faecalis and P.aeruginosa. The result showed that agar well diffusion showed a strong antibacterial activity against P.aeruginosa while there was no significant difference between agar well diffusion method and filter paper disc method against E. faecalis. The antagonistic activity of its bacteriocin suggests its potential as an alternative to antibiotics for combating bacterial infections. However, further investigations are warranted to comprehensively evaluate the antibacterial activities of L. helveticus DF and its base.
None.
The current study is derived from the thesis of a medical student and approved by the Ethical Approval Committee of the Mustansiriyah University, Baghdad, Iraq (Ref.:BCSMU/1221/0008M).
All authors contributed to the conception and design of the study, analysis of data, statistical analysis, preparation, editing, and reviewing of the article.
None.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2023/06/21 | Accepted: 2023/09/1 | ePublished: 2023/09/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |