BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2145-en.html
The genus Proteus involves a diverse group of gram-negative bacilli within the Enterobacteriaceae family, characterized by their rod-shaped morphology. These bacteria are ubiquitous in natural environments, including water and soil (1, 2). Among the diseases associated with this bacterial family are bacteremia, wound infections, burns, and nosocomial catheter-related urinary tract infections. The latter infections involve the colonization of pathogenic bacteria in the urethra, kidney, and bladder, leading to symptoms such as itching and inflammation, often resulting in urethritis and pyelonephritis. Key pathogens responsible for these infections include Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Proteus species, notably Proteus mirabilis and Proteus vulgaris (3, 4).
Proteus species, including P. vulgaris, have drawn attention due to their ability to develop extensive drug resistance (MDR & XDR) against a wide range of antibiotics, driven by the emergence of virulence-resistant genes within their genomic DNA. These genes are located on chromosomal and extra-chromosomal DNA elements, such as plasmids and mobile genetic elements. Additionally, Proteus species exhibit a high capacity for biofilm production, facilitated by specific biofilm-resistant genes. This biofilm formation enhances their virulence, particularly in chronic infections, rendering these bacteria challenging to treat (5, 6).
Proteus is responsible for Hospital-acquired UTIs in catheterized patients admitted to intensive care units of different Hospitals more than any other Enterobacteriaceae. Urinary tract infections by these bacteria occur due to bacterial movement through the catheter sheath. Other factors that increase the risk of UTI by Proteus species, like female patients, improper catheter cleaning, or long catheterization duration, all these factors may lead to bacterial production of urease enzyme and other virulence factors, which in turn increase antibiotic resistance of bacteria to many drugs, such as Proteus vulgaris which is a significant human pathogen, exhibits various modes of infection and transmission to different sites within the human body, including the urethra, vagina, wounds, sputum, urine, blood and feces. It is pivotal in triggering nosocomial infections in patients with indwelling urinary catheters (7, 8).
Moreover, Proteus species, especially P. vulgaris, have developed new β-lactamase enzymes that confer resistance to β-lactam antibiotics and subsequently to a broad spectrum of antibiotics. This has resulted in a substantial increase in multiple drug-resistant (MDR) cases, particularly in urinary tract infections and other sources of infection (9).
The emergence of extensively drug-resistant (XDR) Proteus species, notably Proteus mirabilis and Proteus vulgaris, can be attributed to acquiring new genetic determinants. These genes equip the bacteria with high resistance levels and the ability to form thick biofilm layers in vivo at various infected sites, such as wounds, urethra, and vaginal tracts, particularly in catheterized patients (10-12). Bacterial biofilms comprise multiple species of microbes that communicate and collaborate to form a complex extracellular polymer matrix composed of polysaccharides, lipids, and proteins. This matrix enhances the adhesion of bacteria to surfaces, including catheters, exacerbating infections (13-15).
Proteus species employ various virulence factors, including swarming phenomena, where bacterial cells elongate into short rods and move collectively from liquid to solid media. Additionally, they produce extracellular enzymes, pili, and the urease enzyme, all of which contribute to their virulence (2, 16, 17). The genotypic characterization and investigation of Proteus species, notably P. vulgaris, is crucial in identifying virulence-resistant genes on bacterial chromosomes and extra-chromosomal elements responsible for antibiotic resistance and increased infection pathogenicity. These genes are linked to determinants such as swarming phenomena, enzyme production (including genes accountable for urease production like UreA, UreB, and UreC), lipopolysaccharide lipid-A antigens and other factors facilitating attachment and invasion of host cells, thus promoting disease establishment (18-20).
The current study employed phenotypic and genotypic techniques to investigate P. vulgaris and its resistant strains isolated from different clinical sources of patients with chronic infections. Our determinants of interest included antibiotic resistance, biofilm formation assays, biochemical tests, complete DNA extraction, purification, and the amplification of UreC and blaCTX-M genes using PCR molecular techniques.
This study aimed to shed light on the mechanisms underlying Proteus vulgaris's multidrug resistance, addressing a critical issue in urinary tract and nosocomial infections. Our study laid the foundation for improved diagnostic and therapeutic strategies to combat these challenging infections.
Sample Collection
In the present cross-sectional study, 300 clinical samples were taken from various sources, including urine, blood, sputum, wound, and vaginal swabs. These samples were collected from patients who attended the Intensive care units (ICU) of ten different Baghdad hospitals by rotation over six months from December 2021 to June 2022, including Baghdad Teaching Hospital in the Medical City and Ibn Sina Hospital. The patients suffered from severe burns and chronic diseases, such as Diabetes mellitus, accompanied by severe catheterized urinary tract infections.
Bacteriological Culture and Identification
All clinical samples were cultured on specialized bacteriological culture media, including blood agar and MacConkey agar, following the manufacturer's guidelines (Salucea VOF Dutch technology in life science). These culture plates were then incubated at 37°C in a laboratory incubator. Proteus vulgaris colonies on MacConkey's agar showed non-lactose fermentation while swarming phenomena were observed on blood agar (Figure 1).
Figure 1. Proteus spp. Colonies with swarming growth on blood agar (a) and non-lactose fermenter Proteus spp. on MacConkey agar (b)
Biochemical Testing
Biochemical tests were performed on all P. vulgaris isolates, including oxidase, catalase, urease, H2S production, motility, citrate, and indole tests. These tests aimed to differentiate between indole-positive P. vulgaris and indole-negative P. mirabilis and exclude other Proteus species (21-23).
Antimicrobial Sensitivity Testing
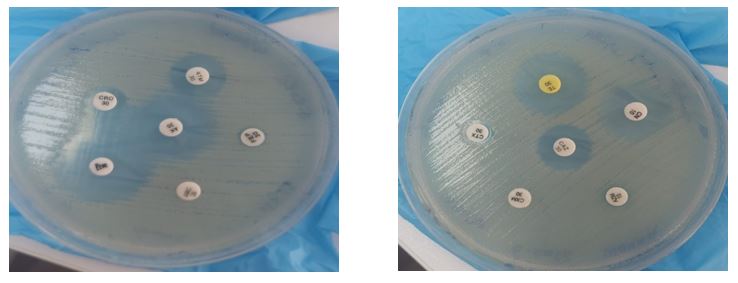
The antibiotic susceptibility of all P. vulgaris isolates was determined using Kirby-Bauer disc diffusion methods with 20 different antibiotic discs from Biomerieux (USA). The isolates were standardized to 0.5 McFarland turbidity standard containing 1x108 CFU/ml for uniformity. The antibiotic discs were placed on Muller Hinton agar plates, then incubated at 35°C for 18 hours (Figure 2). The specific antibiotics used in this study were Amikacin, Gentamycin, Ampicillin, Imipenem, Meropenem, Tetracycline, Tobramycin, Doxycycline, Ciprofloxacin, Levofloxacin, Azithromycin, Piperacillin, Cefotaxime, Trimethoprim/sulfamethoxazole, Calvulanic acid, Cefoxitin, Ceftriaxone, Aztreonam, Cefepime, and Chloramphenicol. The antibiotic susceptibility was determined following CLSI criteria (24-26).
Figure 2. Antibiotic sensitivity tests of selected antibiotic discs on cultured Proteus vulgaris isolates; this is a simplified and illustrative image only to express the sensitivity test for the isolated bacteria, where the zone of inhibition was measured using a special graduated ruler and compared with the standard results of the CLSI reference.
Biofilm Assay Method
Biofilm formation by P. vulgaris isolates was quantitatively assessed using the tissue culture plate method with modifications (Figure 3); this figure about biofilm assay test in tissue culture plate method is needed to demonstrate the initial and final step of bacterial biofilm formation in vitro depending on its color changes. Isolates were cultured in a Tryptic Soy Broth (TSB) medium with the addition of 2% glucose to enhance biofilm production. After incubation, the biofilm formed in microtiter plates was measured by optical density (OD) at 580 nm wavelength using an ELISA microtiter plate reader (27). Based on absorbance values, biofilm strength was categorized as weak, moderate, or firm. The cutoff limit of P. vulgaris biofilm is listed in (Table 1).
Figure 3. Biofilm assay for cultured isolates in the initial step (a) and final step (b)
Table 1. Cutoff limit of P. vulgaris biofilm
| Biofilm strength | Cutoff | Weak biofilm | Moderate biofilm | Strong biofilm |
| O.D. limit absorbance | ≤100 nm | 100-149 nm | 150-200 nm | >200 nm |
Molecular Detection of Proteus vulgaris
Genomic DNA was extracted and purified from 100 positive cultures of Proteus vulgaris isolates. This was followed by detecting resistance genes (UreC and blaCTX-M) on the whole bacterial chromosomes using conventional PCR techniques with designed primers from Alpha DNA Canada.
Genomic DNA Extraction and Purification
Genomic DNA was extracted from P. vulgaris isolates grown in brain heart infusion broth (BHI) at 37°C for 24 hours. DNA purity and concentrations were determined using a Nanodrop instrument, ensuring an optical density (OD) ratio of ~1.8 at 260/280 nm (Table 2).
Table 2. DNA purity of the selected samples by Nanodrop
| Sample ID | Abs. 260 | Abs. 280 | 260/280 | Conc. (ng/ul) | Sample Type |
| 1 | 1.164 | 0.607 | 1.92 | 58.2 | dsDNA |
| 2 | 1.817 | 0.994 | 1.83 | 90.9 | dsDNA |
| 3 | 1.620 | 0.824 | 1.97 | 81.0 | dsDNA |
| 4 | 1.650 | 0.852 | 1.94 | 82.5 | dsDNA |
| 5 | 2.761 | 1.736 | 1.59 | 138.0 | dsDNA |
Amplification of Target Genes by Conventional PCR
Conventional PCR was used to amplify resistance genes in P. vulgaris, specifically the UreC (263 bp) and blaCTX-M (550 bp) genes, based on previous studies were used as references in this study for primer design (Samira Fattah Hamid et al., Kamil and Jarjes), the nucleotide sequence of each type of primer for each gene was sent to a private Company in Baghdad. This company deals with Alpha DNA Company in Canada to design the primers for the study for 0.9$ for each nitrogenous base (28-30). DNA and PCR products were analyzed by 1% agarose gel electrophoresis and visualized under a UV-trans illuminator (Table 3).
Table 3. PCR-specific primer sequences of amplified target genes
| Target gene | Nucleotide sequences (5' to 3') | Amplicon (bp) | Reference | |
| UreC | F: | CGTTTGCGATGGCAAGTACAAGTAAG | 263 | (28) |
| R: | GCAAATTGAGTGACTTTGGCTGGACC | |||
| BlaCTX-M | F: | CGCTTTGCGATGTGCAC | 550 | (30) |
| R: | ACCGCGATATCGTTGGT | |||
Statistical analysis was carried out using SPSS version 22.0. The frequency of Proteus vulgaris isolated from different clinical samples was analyzed using chi-square tests. p-values were used to determine statistical significance, with p≤0.05 indicating significant, p>0.05 non-significant, and p ≤ 0.01highly significant.
The study included 100 positive P. vulgaris isolates obtained from various clinical sources of patients of different ages and genders. The most prevalent rate of isolates (40%) was found in the age group 40-49 years, comprising 40 females and 10 males. A similar distribution was observed for P. mirabilis isolates in the same age group, with a non-significant correlation (p > 0.05) (Table 4).
Table 4. Demographic distribution of isolates according to age groups
| Age range | Gender | P. vulgaris Isolates |
P. mirabilis Isolates |
p-value | |
| Male | Female | ||||
| 20-29 | 20 | 30 | 10 (10%) | 4 (8%) | >0.05 NS* |
| 30-39 | 22 | 28 | 12 (12%) | 5 (10%) | |
| 40-49 | 10 | 40 | 40 (40%) | 20 (40%) | |
| 50-59 | 15 | 35 | 15 (15%) | 10 (20%) | |
| 60-69 | 25 | 25 | 15 (15%) | 7 (14%) | |
| >69 | 27 | 23 | 8 (8%) | 4 (8%) | |
| Total | 119 (40%) | 181 (60%) | 100 (100%) | 50 (100%) | |
* p-value >0.05 is considered statistically non- significant (NS)
Among the 300 clinical samples, 150 showed positive cultures for Proteus species (100 positive for P. vulgaris and 50 positive for P. mirabilis). Urine samples constituted the predominant source, with 50% positive isolates for P. vulgaris and 22% positive isolates for P. mirabilis. Wound pus was the second most frequent source, accounting for 30% of positive P. vulgaris isolates and 18% of positive P. mirabilis isolates, with statistically non-significant differences (p > 0.05) (Table 5).
Table 5. Distribution of Proteus species isolated from different clinical samples
| Specimens | Frequency | P. vulgaris | P. mirabilis | Total culture | p-value |
| Urine | 100 | 50(50%) | 22(22%) | 72(48%) | >0.05 NS* |
| Wounds | 50 | 30(30%) | 18(18%) | 48(32%) | |
| Vagina | 50 | 10(10%) | 5(5%) | 15(10%) | |
| Blood | 50 | 8(8%) | 4(4%) | 12(8%) | |
| Sputum | 50 | 2(2%) | 1(1%) | 3(2%) | |
| Total | 300 | 100(100%) | 50(50%) | 150(100%) |
* p-value >0.05 considered statistically non-significant (NS)
Biochemical screening tests demonstrated that P. vulgaris isolates showed positive indole results, while P. mirabilis showed negative indole results. The isolates of P. vulgaris showed positive citrate test results, while the P. mirabilis species showed variable citrate test results, which served to distinguish between the two Proteus species in the study (Table 6).
Table 6. Biochemical tests of Proteus species isolates
| Tests | Indole | VP | Citrate | MR | Urease | Oxidase | Catalase |
| P. vulgaris | Positive | Negative | Positive | Positive | Positive | Negative | Positive |
| P. mirabilis | Negative | Negative | Variable | Positive | Positive | Negative | Positive |
The antibiotic resistance analysis of 150 positive Proteus species isolates from different clinical sources (100 P. vulgaris and 50 P. mirabilis) revealed high antibiotic resistance among P. vulgaris isolates, with resistance observed against approximately 16 out of 20 antibiotic discs, indicating multidrug resistance (MDR) and possible extensive drug resistance (XDR). P. vulgaris isolates exhibited higher antibiotic resistance compared to other Proteus species in the study, with complete resistance (100%) to clavulanic acid, 95% to doxycycline, 91% to cefoxitin, 90% to ampicillin, tetracycline and cefepime. The differences in antibiotic resistance between the two Proteus species were highly significant (p < 0.001) (Table 7).
Table 7. Antibiotic resistance percentage of Proteus species
| Antibiotic | Dose (µg) | Resistance percentage | p-value | |
| P. vulgaris | P. mirabilis | |||
| Amikacin (AK) | 30 | 77% | 57% | 0.001HS* |
| Gentamycin (GM) | 10 | 82% | 61% | |
| Ampicillin (AM) | 15 | 90% | 55% | |
| Imipenem (IPM) | 10 | 12% | 3% | |
| Meropenem (MEM) | 10 | 11% | 1% | |
| Tetracycline (TET) | 30 | 90% | 38% | |
| Tobramycin (TM) | 15 | 80% | 28% | |
| Doxycycline (DOX) | 25 | 95% | 80% | |
| Ciprofloxacin (CIP) | 10 | 72% | 40% | |
| Levofloxacin (LEV) | 10 | 55% | 22% | |
| Azithromycin (AZM) | 15 | 18% | 10% | |
| Piperacillin (PIP) | 100 | 22% | 9% | |
| Cefotaxime (CTX) | 30 | 88% | 64% | |
| Cotrimox (COT) | 10 | 77% | 46% | |
| Clavulanic acid (CA) | 15 | 100% | 90% | |
| Cefoxitin (CX) | 30 | 91% | 70% | |
| Ceftriaxone (CTR) | 30 | 73% | 65% | |
| Aztreonam (AZM) | 10 | 49% | 20% | |
| Cefepime (CEF) | 30 | 90% | 58% | |
| Chloramphenicol (CHL) | 30 | 79% | 70% | |
*p-value ≤0.01 is considered highly significant (HS)
Biofilm formation ability among all Proteus species isolates was assessed, indicating a high capacity for strong biofilm production, particularly in P. vulgaris isolates (70%) and P. mirabilis (50%). This association was highly significant (p = 0.01) (Table 8).
Table 8. Biofilm production of all Proteus isolates regarding cutoff value (≤ 100 nm)
| Biofilm strength | Non-biofilm | Weak | Moderate | Strong | Total culture |
| P. vulgaris | 5 (5%) | 8 (8%) | 17 (17%) | 70 (70%) | 100 (100%) |
| P. mirabilis | 7 (14%) | 8 (16%) | 10 (20%) | 25 (50%) | 50 (100%) |
| p-value | 0.01HS* | ||||
*p-value ≤0.01 is considered highly significant (HS)
All 20 types of traditional antibiotics in the study were intended to determine these isolated bacteria' resistance rate to several types of antibiotics according to CLSI standards. Therefore, they were used to compare the rate of resistance of bacteria to antibiotics (MDR and XDR) with other determinants, such as the strength of the bacterial biofilm formation. The study also showed a significant association between solid biofilm formation and increased antibiotic resistance among Proteus species, as 70% of P. vulgaris isolates exhibited strong biofilm production, correlated with high resistance (70-100%) to 14 antibiotics. In contrast, only 10% of strong biofilm producers were observed among P. mirabilis isolates, and these isolates exhibited high resistance (70-100%) to only four antibiotics. This association was highly significant (p < 0.000) (Table 9).
Table 9. Association between antibiotic resistance and strong biofilm production for all Proteus isolates
| Antibiotic R % | No. antibiotics | P. vulgaris No. (%) | P. mirabilis No. (%) | p-value |
| (50-69) % | 20 | 1 (5%) | 6 (30%) | 0.00 HS* |
| (70-100) % | 20 | 14 (70%) | 4 (10%) | |
| (R/biofilm) % | --- | 15 (75%) | 10 (40%) |
*p-value ≤0.01 is highly significant (HS), R= resistance.
PCR analysis of targeted genes in 100 Proteus vulgaris isolates revealed that 75% had positive UreC genes and 50% had positive CTX-M genes when amplified by PCR and analyzed by agarose gel electrophoresis. Among the clinical sources, urine samples showed the highest UreC (50%) and CTX-M (33%) genes distribution. Wound pus samples contained approximately 22% UreC and 12% CTX-M genes with a non-significant difference in gene distribution (p > 0.05) (Figures 4 and 5) (Table 10).
Table 10. PCR of P. vulgaris target genes according to sample types
| Sample | Frequency | UreC [No. (%)] | BlaCTX-M [No. (%)] | p-value |
| Urine | 50 | 50(50%) | 33(33%) | 0.71 NS* |
| Wounds | 30 | 22(22%) | 12(12%) | |
| Vagina | 10 | 2(2%) | 3(3%) | |
| Blood | 8 | 1(1%) | 2(2%) | |
| Sputum | 2 | 0 | 0 | |
| Total | 100 | 75(75%) | 50(50%) |
* p-value >0.05 is considered statistically non- significant (NS)

Figure 4. Amplification of UreC genes of P. vulgaris DNA isolated from urine samples. Lane L: Molecular Ladder (100 bp), Lane 1-7: PCR products of amplified 263 bp UreC Genes, Lanes 8-12: Negative PCR products.
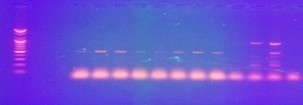
Figure 5. Amplification of Beta-Lactamase CTX-M genes in P. vulgaris DNA isolated from urine samples. Lane L: Molecular Ladder (100 bp), Lanes 1-8: PCR products of amplified 550 bp CTX-M genes, Lanes 9-12: Negative PCR products.
Proteus species are globally recognized as opportunistic pathogens responsible for severe inflammatory urinary and nephritis diseases. They rank as the third most common cause of urinary tract infections (UTIs), closely behind Escherichia coli and Klebsiella (19, 31, 32). In our study, which included 300 samples collected from diverse clinical sources of medical significance, we aimed to shed light on several critical aspects of Proteus infections. Our findings enhanced our understanding of these pathogens and offered valuable insights into their clinical implications.
Results of our study revealed a notable gender distribution, with males comprising 40% of the participants and females comprising 60%. This discrepancy in gender distribution was different from the study by P. Snega Priya, who found that males accounted for 65% and females 35% among 100 study samples, which can be attributed to our random sample collection approach (33). While gender distribution was not the primary focus of our study, it is worth mentioning that such variation can influence susceptibility to infections and potentially affect the study outcomes. Further investigations are required to explore the underlying factors contributing to this gender distribution discrepancy and its potential implications for Proteus infections.
The patients’ ages ranged between 20-69 years in our study and were categorized into six age groups. It was shown that Proteus vulgaris was the predominant isolate in the age groups 40-49 and 50-59 years. These findings agreed with the results of Pal and Sharma (34). Their study demonstrated a higher prevalence of Proteus species among individuals aged 20-49, accounting for approximately 75% of their 101 samples (34). This suggests that specific age groups may be more susceptible to nosocomial contamination, a phenomenon worthy of further investigation. Age-related patterns are crucial in the epidemiology of infectious diseases, and understanding these patterns is pivotal for designing targeted preventive measures.
In our study, Proteus species were predominantly isolated from clinically significant samples, with urine and wound pus being the most common sources. Proteus vulgaris was the most frequently isolated species in our study, particularly in urine samples (50%) and wound pus (30%). These findings are the same as those of Shamsuzzaman et al., who reported a high incidence of Proteus species in urine (72%) and wound samples (72.9%) (35). However, these results were contrary to the findings of Nita Pal, where the wound samples were the primary source of Proteus isolates (16%) (34). These disparities emphasize the importance of urine as a significant reservoir of P. vulgaris, particularly in urinary tract infections (UTIs).
Our study revealed a concerning antibiotic resistance pattern in Proteus vulgaris isolates, characterized by multi-drug resistance (MDR). High antibiotic resistance levels to many antibiotics were reported in this study due to these bacteria being gram-negative and having several genetic and antigenic determinants on their cell wall and chromosomal DNA like lipopolysaccharide, antibiotic resistance gene to cefotaxime and other virulence factors that contribute to their high rate of multiple antibiotic resistance. Among the 20 narrow and broad-spectrum antibiotics tested, P. vulgaris isolates showed resistance to 14 types (70%). The most resistant antibiotics in our study were clavulanic acid (100%), doxycycline (95%), cefoxitin (91%), ampicillin (90%), tetracycline (90%) and cefepime (90%). However, Proteus mirabilis isolates demonstrated lower resistance rates, with only four antibiotics (10%) exhibiting high resistance. These findings aligned with the study conducted by Fm and Se, who highlighted MDR patterns in Proteus species and showed that the high resistance rates were the antibiotics tetracycline (100%), ampicillin (85%), cotrimoxazole (78.8%) and chloramphenicol (72.2%) (36). However, our results diverged from those of Habibi et al., who reported lower resistance rates in Proteus species to chloramphenicol (25%), tetracycline (33%), ciprofloxacin (38%) and amikacin (12%), among others (33). These disparities underscore the variability in antibiotic resistance patterns among Proteus species and emphasize the need for further studies to elucidate contributing factors.
Biofilm formation, a critical virulence factor, was evaluated in our study. Proteus vulgaris exhibited a notably higher capacity for strong biofilm production (70%) than Proteus mirabilis (50%). Biofilm formation is known to contribute to antibiotic resistance, and our results align with a previous study by Wasfi and El-Rahman et al., who revealed a strong correlation between antibiotic resistance and strong biofilm formation ability in Proteus isolates (37). These findings underscore the clinical implications of biofilm production in Proteus species, particularly P. vulgaris, and its potential impact on antibiotic treatment outcomes.
Our genetic analysis focused on UreC and β-lactamase CTX-M genes in Proteus vulgaris, revealing their presence in various clinical samples. UreC genes were detected in 75% of isolates, while 50% of the isolates contained CTX-M genes, with a higher prevalence in urine samples. These genetic characteristics are consistent with the study by Passat et al., who demonstrated the presence of β-lactamase genes for cefotaxime resistance (CTX-M) in Proteus species (38). Furthermore, our study detected the UreC gene in all bacterial DNA isolated from different clinical samples, which aligns with the findings of Bahashwan and El Shafey et al. (26). However, these results did not agree with the study by Hamid and Taha, who reported a lower prevalence of β-lactamase CTX-M genes in Proteus species (39). Virulence-resistant genes of P. vulgaris in this study, like CTX-M and others, are very important in the acquisition of this bacteria to many antigenic determinants on its DNA that contribute to its multiple drug resistance of many antibiotics used for the treatment of UTIs. These genetic findings underscore the clinical relevance of specific genes in Proteus infections and warrant further investigations into their impact on patient outcomes and treatment strategies.
Our study highlighted significant multi-drug resistance (MDR) in Proteus species, particularly P. vulgaris, in patients with urinary tract and other infections, primarily in urine and wound samples. Biofilm formation, notably in P. vulgaris, contributed to antibiotic resistance. Effective antibiotics, such as imipenem, meropenem, and piperacillin, remain crucial for treatment. PCR analysis revealed prevalent UreC and CTX-M resistant genes in P. vulgaris, particularly in urine samples. These findings underscore the use of other types of effective antibiotics in treating such infections, recognizing biofilm-related resistance, and further research to address the clinical implications of these resilient pathogens.
The author is thankful to the Ministry of Health, Iraq, the consultant clinic and laboratories of Baghdad Teaching Hospital in Medical City, and especially to Ibn-Sina Hospital for their cooperation in accomplishing this study.
Conflicts of Interest
The authors declare no conflict of interest.
None.
Received: 2023/06/22 | Accepted: 2023/08/29 | ePublished: 2023/11/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |