BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2125-en.html


 , Ebrahim Barzegari2
, Ebrahim Barzegari2 

 , Lida Lotfollahi3
, Lida Lotfollahi3 

 , Reza Jafari4
, Reza Jafari4 

 , Bizhan Nomanpour5
, Bizhan Nomanpour5 

 , Mahsa Rasekhian6
, Mahsa Rasekhian6 


2- Medical Biology Research Center, Health Technology Institute, Kermanshah University of Medical Sciences Kermanshah, Iran
3- Department of Microbiology and Virology, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
4- Cellular and Molecular Research Center, Cellular and Molecular Medicine Research Institute, Urmia University of Medical Sciences, Urmia, Iran
5- Department of Microbiology, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran ,
6- Pharmaceutical Sciences Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
Antibiotic resistance is recognized as a major threat to global health that can result in increased morbidity and mortality (1). In 2019, antimicrobial resistance (AMR) was declared one of the ten major threats to global health by the World Health Organization (WHO) (2). Similarly, the Centers for Disease Control and Prevention (CDC) states that each year at least 2.8 million new cases of antibiotic-resistant infection happen in the United States, causing 35,000 deaths from AMR (3). Since 2007, a significant increase in incidences of AMR infections has been observed (4). Reports estimate the global death toll from AMR to be around 700,000 and AMR-related deaths are expected to rise to 10 million annually by 2050 (5, 6). Such mortality rates bring AMR shoulder to shoulder with cancer-related deaths (3). Factors including but not limited to consumption of inappropriate doses of antibiotics and improper use of antibiotics to treat viral infections have been reported as main contributors to the emergence and spread of resistant microorganisms (7). To achieve an effective solution to the growing problem of antibiotic resistance, it seems necessary to design strategies at the global level. Designing effective vaccines to prevent the occurrence of AMR infections appeals as an effective strategy in tackling the spread of such infections (3, 8, 9). WHO On February 27, 2017, published the first list of antibiotic-resistant pathogens, called ESKAPE, which were assigned the highest "priority status" because they pose the greatest threat to human health. The members of this list were selected based on the urgency and need for new antibiotics (10). Pseudomonas aeruginosa; an aerobic gram-negative bacterium, is classified as one of the most common nosocomial pathogens. PA infection especially affects patients hospitalized in burn and ICU departments. P. aeruginosa is known as the causative agent in a wide range of diseases, including bacteremia, pneumonia, burn wounds, systemic infections in patients with suppressed immunity and cancer, and chronic infection in people with cystic fibrosis (11, 12). It seems that vaccination strategies can be used as a suitable option to prevent or treat AMR infections. Nevertheless, currently, there are no licensed vaccines available against AMR P. aeruginosa infection (12-14). Reverse vaccinology (RV) is a cost-effective, time-saving, and accurate approach to vaccine design compared to conventional methods. Successful examples of application of RV approaches in vaccine design have been reported previously (15, 16). RV includes detailed genomic and proteomic assessment of a specific pathogen to find optimal vaccine candidates regarding surface exposure, immunogenic potential, abundance, number of transmembrane helices, and involvement in virulence (17). A successful use of reverse vaccinology tools to predict COVID-19 vaccine candidates have had a great effect on the 2019 pandemic (18). Vaxign2 is the second generation of the first web-based vaccine design program using reverse vaccinology and machine learning (19). In addition, clinical and laboratory experts can adopt machine learning (ML) in disease diagnosis, in laboratory applications and tools (20). Gram-negative bacteria such as PA are restricted by two concentric lipid bilayer membranes. The inner membrane, which is solely composed of phospholipids (mainly phosphatidylethanolamine), and the outer membrane, in contradiction to the inner membrane, is highly asymmetric, with about 50% protein component. Outer membrane proteins (OMPs) are found either in the form of integral membrane proteins or as lipoproteins that are anchored to the membrane employing N-terminally attached lipids. According to pseudomonas.com database (https://www.pseudomonas.com/), Pseudomonas aeruginosa (PAO1 strain) has 194 outer membrane proteins (OMPs). Although the exact function and expression of a large number of PA OMPs are still unknown, it has been shown that OMPs contribute significantly to the structure of the bacterial cell surface. Accordingly, the components of OMPs are attractive for the development of clinical vaccines due to their presence on the cell surface and conserved antigenic domains in various strains of P. aeruginosa (21). In this study, we used the RV approach to evaluate PA OMPs to introduce a viable candidate for vaccine development against PA OMPs.
Collection of P. aeruginosa Genome and Outer Membrane Proteins (OMPs) Sequences and Vaxign2 Calculation of Sequence-Derived Features
The Pseudomonas Genome Database (http://pseudomonas.com), contains 1071 records of complete genomes. In the first step, 121 records of PA that were isolated from the human host were selected. Subsequently, only 58 records that were isolated from the host with a specific disease were selected for further analysis of OMPs by the Vaxign2 database (Vaxign2 is publicly accessible at http://www.violinet.org/vaxign2 ). In the next step, all of the outer membrane proteins of each strain were extracted by searching the subcellular localization section of the Pseudomonas genome database. The RefSeq accession number of each protein was then used to extract additional data from NCBI. Each protein ID number then was submitted to vaxign2 to obtain antigenic properties including transmembrane domain prediction, adhesion probability, and homology to human proteins. Vaxign2 uses PSORTb2.0, TMHMM, and SPAAN algorithms to predict subcellular localization and analyze transmembrane helix topology and adhesion probability (Figure 1).
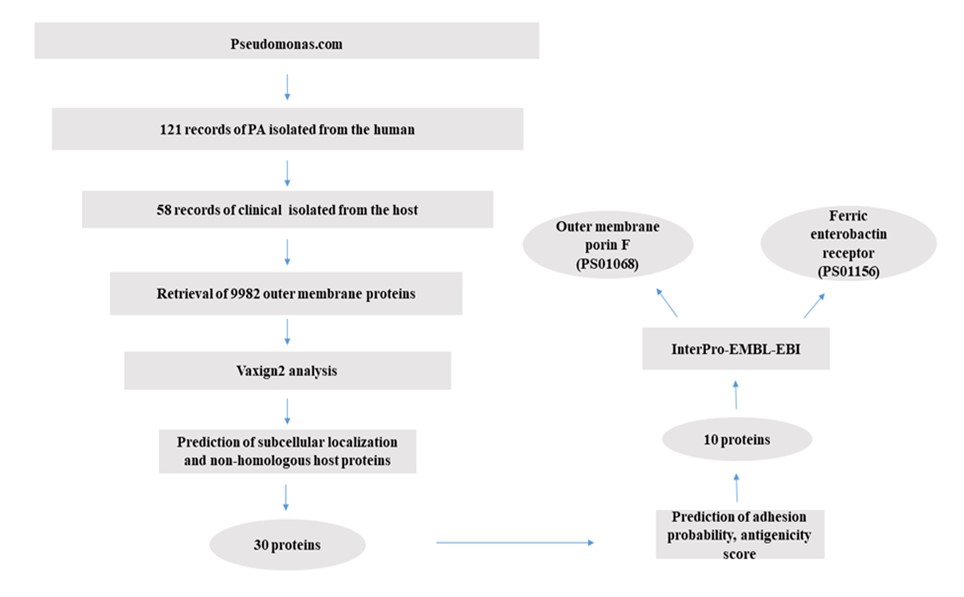
Figure 1. Vaxigen2 workfellow diagram for prediction of PA OMP antigen candidates.
An imperative step in selecting an appropriate vaccine target is to predict the subcellular localization of an infectious agent proteome. Because the genome of P. aeruginosa was not in the list of pre-calculated genomic groups, the analysis was performed in the Dynamic Vaxign2 analysis section instead of applying the Vaxign2 query program. PSORTb in Vaxign2 is designed to predict bacterial protein localization. Using TMHMM v2.0, the topology of surface-exposed and secreted proteins was determined. Proteins with more than one transmembrane helix should be excluded from the final list. Homology and host similarity analysis of each OMP to human proteins was performed through BLAST by Vaxign2.
Prediction of Adhesion Probability, Antigenicity Score, and Conserved Domains
Since cell surface components are considered more suitable vaccine targets, Vaxign2 calculated adhesion using SPAAN was used for selecting OMP vaccine candidates in this study. Antigenicity scores of vaccine candidate proteins were calculated by the VaxiJen v2.0 server. Antigen candidates with antigenicity scores higher than 0.4 are usually considered. For protein functional description, the CLC main workbench was used to describe a number of physicochemical parameters such as isoelectric point (pI), molecular weight, estimated half-life, GRAVY value, aliphatic index, and instability index. InterPro-EMBL-EBI database (https://www.ebi.ac.uk/interpro/) was used to search the conserved domains present in candidate proteins.
Fifty-eight clinical isolates and 9982 outer membrane protein Sequences were retrieved for Vaxign2 Calculation of Sequence-Derived Features. In this study, 58 records of complete genomes for PA that were isolated from known diseases of patients were selected. The number of genes ranged from 5643 to 6894. The number of annotated OMPs on the other hand ranged from 152 to 333 (Table 1). All annotated OMPs for each strain were used to identify P. aeruginosa vaccine-candidate antigens in Vaxign2 analysis. The RefSeq accession number of each protein was used for collecting additional data from NCBI.
Table 1. Fifty-eight clinical isolates of Pseudomonas aeruginosa used for reverse vaccinology (RV) study.
| Number | Strain | Number of Genes | Number of OMPs | Sample Accession | Host Disease | Isolation Source |
| 1 | 97 | 6575 | 333 | SAMN07692776 | UTI | urine |
| 2 | PA34 | 6396 | 329 | SAMN08435059 | Microbial Keratitis | eye |
| 3 | PAO1 | 5688 | 194 | SAMN02603714 | - | wound |
| 4 | CI27 | 6402 | 180 | SAMN13781155 | Cystic fibrosis | physical |
| 5 | Pa58 | 6752 | 180 | SAMN05020321 | ventilator-associated pneumonia | bronchial washing |
| 6 | CF39S | 6780 | 176 | SAMN13226654 | Cystic Fibrosis | lung |
| 7 | isolate M37351 | 6322 | 176 | SAMN02894351 | cancer | - |
| 8 | Pa124 | 6558 | 176 | SAMN05020323 | ventilator-associated pneumonia | bronchial washing |
| 9 | UCBPP-PA14 | 5977 | 176 | SAMN02603591 | burn | burn wound |
| 10 | Pa127 | 6644 | 175 | SAMN05020324 | ventilator-associated pneumonia | bronchial washing |
| 11 | MRSN12280 | 6679 | 174 | SAMN08776459 | Wound | Sacrum |
| 12 | NCGM257 | 6628 | 173 | SAMD00020552 | urinary tract infection | Midstream urine |
| 13 | Y31 | 6402 | 172 | SAMN09469677 | pneumonia | sputum |
| 14 | AZPAE15042 | 6240 | 171 | SAMN03105739 | urinary tract infection | - |
| 15 | SCV20265 | 6380 | 171 | SAMN02415141 | cystic fibrosis | lung |
| 16 | isolate F30658 | 6622 | 170 | SAMN02894357 | cancer | - |
| 17 | F63912 | 6196 | 169 | SAMN02894356 | cancer | missing |
| 18 | Y89 | 6546 | 169 | SAMN09469733 | pneumonia | sputum |
| 19 | E6130952 | 6715 | 168 | SAMN06349407 | respiratory failure | sputum |
| 20 | isolate H47921 | 6249 | 168 | SAMN02894353 | cancer | - |
| 21 | PA_D1 | 6069 | 168 | SAMN04910034 | Ventilator associated pneumonia | Sputum; Early isolate from VAP patient 1 |
| 22 | PA_D2 | 6066 | 168 | SAMN04910045 | Ventilator associated pneumonia | Sputum; Early isolate from VAP patient 2 |
| 23 | PA_D5 | 6087 | 168 | SAMN04910061 | Ventilator associated pneumonia | Sputum; Early isolate from VAP patient 3 |
| 24 | PA_D9 | 6065 | 168 | SAMN04910066 | Ventilator associated pneumonia | Sputum; Late isolate from VAP patient 1 |
| 25 | PA_D16 | 6086 | 168 | SAMN04914381 | Ventilator associated pneumonia | Sputum; Early isolate from VAP patient 4 |
| 26 | PA_D21 | 6063 | 168 | SAMN04914386 | Ventilator associated pneumonia | Sputum; Late isolate from VAP patient 2 |
| 27 | PA_D22 | 6091 | 168 | SAMN04914475 | Ventilator associated pneumonia | Sputum; Late isolate from VAP patient 3 |
| 28 | W60856 | 6380 | 168 | SAMN02894343 | cancer | missing |
| 29 | Y82 | 6718 | 168 | SAMN09469732 | pneumonia | sputum |
| 30 | PA121617 | 6303 | 167 | SAMN05006707 | Respiratory disease | sputum |
| 31 | M1608 | 6014 | 166 | SAMN02894352 | cancer | missing |
| 32 | PA1 | 6054 | 166 | SAMN02603191 | respiratory tract infection | - |
| 33 | F22031 | 6077 | 165 | SAMN02673269 | cancer | pubic bone |
| 34 | X78812 | 5886 | 165 | SAMN02894342 | cancer | missing |
| 35 | F5677 | 6242 | 164 | SAMN02887043 | cancer | urine |
| 36 | Pa1207 | 6894 | 164 | SAMN05020325 | Comunity-adquired pneumonia | blood |
| 37 | T38079 | 6257 | 164 | SAMN02894349 | Cancer | missing |
| 38 | F23197 | 5953 | 163 | SAMN02894358 | cancer | missing |
| 39 | Pa84 | 6138 | 163 | SAMN05020322 | ventilator-associated pneumonia | bronchial washing |
| 40 | PACS2 | 5989 | 162 | SAMN02471994 | cystic fibrosis | - |
| 41 | S86968 | 6480 | 162 | SAMN02894350 | cancer | missing |
| 42 | W45909 | 6288 | 162 | SAMN02894344 | cancer | missing |
| 43 | isolate T52373 | 5643 | 161 | SAMN02894348 | cancer | - |
| 44 | isolate T63266 | 5880 | 161 | SAMN02894347 | cancer | - |
| 45 | LES431 | 6091 | 161 | SAMN02641592 | healthy | isolated from a non-CF parent of a CF patient |
| 46 | VA-134 | 5804 | 161 | SAMN04284690 | burn | Skin wound of burn human patient |
| 47 | W36662 | 6364 | 161 | SAMN02894345 | cancer | missing |
| 48 | ATCC 27853 | 6312 | 160 | SAMN04589231 | nosocomial infections | missing |
| 49 | H27930 | 6042 | 160 | SAMN02894354 | cancer | missing |
| 50 | isolate F9670 | 5987 | 160 | SAMN02894359 | cancer | - |
| 51 | W16407 | 6285 | 160 | SAMN02894346 | cancer | missing |
| 52 | H5708 | 5909 | 159 | SAMN02894355 | cancer | missing |
| 53 | FRD1 | 6179 | 158 | SAMN02732380 | cystic fibrosis | sputum |
| 54 | Pa1242 | 6384 | 158 | SAMN05020326 | Chiari malformation | blood |
| 55 | RP73 | 5864 | 157 | SAMN02603771 | cystic fibrosis | - |
| 56 | DK2 | 5959 | 156 | SAMN02603895 | cystic fibrosis | sputum |
| 57 | AES1M | 5927 | 152 | SAMN11087507 | cystic fibrosis | sputum |
| 58 | AES1R | 5912 | 152 | SAMN11087508 | cystic fibrosis | sputum |
Predicted PA Outer Membrane Protein Vaccine Candidate Based on Genome Sequence Analysis
Since PAO1 is the most commonly used strain for research on P. aeruginosa, the results of the analyses performed by Vaxign2 on this strain were considered for selecting the candidate OMPs. First, 30 proteins with the highest adhesin probability were selected out of 194 OMPs (Table 2). Adhesins are essential for bacterial invasion and have an invaluable role in bacterial infestation. Usually, proteins with adhesion probabilities more than 0.40 show adequate antigenicity. The adhesin probability of these proteins was in the range of 0.916 to 0.625. Subsequently, 10 proteins with the highest Vaxign-ML scores (from 99.9 to 89.9) were selected out of the aforementioned 30 proteins, and, Finally, 10 candidate proteins were examined in terms of Vaxign-ML scores and adhesin probability in other strains (Table 3). The results showed very similar ones to those obtained in PAO1 (Table 4).
Table 2. Predicted vaccine targets based on adhesion probability
| # | Protein Accession | Protein Name | Adhesin Probability | Trans-membrane Helices | Similar Human Protein |
| 1 | NP_249774.1 | ferrichrome receptor FiuA | 0.916 | 0 | - |
| 2 | NP_249869.1 | hypothetical protein PA1951 | 0.892 | 0 | - |
| 3 | NP_253350.1 | glycine-glutamate dipeptide porin OpdP | 0.868 | 0 | - |
| 4 | NP_253244.1 | hypothetical protein PA2057 | 0.85 | 0 | - |
| 5 | NP_253279.1 | porin D | 0.847 | 0 | - |
| 6 | NP_249979.1 | pyrophosphate-specific outer membrane porin OprO | 0.843 | 0 | - |
| 7 | NP_252051.1 | outer membrane protein OprG | 0.834 | 0 | - |
| 8 | NP_253204.1 | anaerobically-induced outer membrane porin OprE | 0.801 | 0 | - |
| 9 | NP_249161.1 | hemagglutinin | 0.799 | 0 | - |
| 10 | NP_250641.1 | glycine betaine transmethylase | 0.797 | 0 | - |
| 11 | NP_253191.1 | outer membrane porin F | 0.757 | 0 | - |
| 12 | NP_250747.1 | hypothetical protein PA0165 | 0.745 | 0 | - |
| 13 | NP_249649.1 | hypothetical protein PA3422 | 0.732 | 0 | - |
| 14 | NP_251970.1 | TonB-dependent receptor | 0.725 | 1 | - |
| 15 | NP_252756.1 | TonB-dependent receptor | 0.725 | 0 | - |
| 16 | NP_248982.1 | ferric enterobactin receptor | 0.719 | 1 | - |
| 17 | NP_248731.1 | protease LasA | 0.715 | 1 | - |
| 18 | NP_251772.1 | second ferric pyoverdine receptor FpvB | 0.702 | 0 | - |
| 19 | NP_250468.1 | lactonizing lipase | 0.701 | 0 | - |
| 20 | NP_248855.1 | hypothetical protein PA0982 | 0.696 | 0 | - |
| 21 | NP_252112.1 | hypothetical protein PA2760 | 0.695 | 0 | - |
| 22 | NP_253364.1 | hypothetical protein PA4897 | 0.693 | 0 | - |
| 23 | NP_251958.1 | ferrichrome receptor FiuA | 0.677 | 0 | - |
| 24 | NP_251378.1 | hypothetical protein PA1951 | 0.674 | 0 | - |
| 25 | NP_250562.1 | glycine-glutamate dipeptide porin OpdP | 0.673 | 0 | - |
| 26 | NP_252857.1 | hypothetical protein PA2057 | 0.656 | 0 | - |
| 27 | NP_251552.1 | porin D | 0.65 | 1 | - |
| 28 | NP_249673.1 | pyrophosphate-specific outer membrane porin OprO | 0.627 | 0 | - |
| 29 | NP_251450.1 | outer membrane protein OprG | 0.627 | 0 | - |
| 30 | NP_253584.1 | anaerobically-induced outer membrane porin OprE | 0.625 | 0 | - |
Table 3. Proteins with the highest antigenicity scores extracted from OMPs with the highest adhesion probability
| # | Protein Accession | Protein Name | Vaxign Score | Length |
| 1 | NP_253204.1 | iron transport outer membrane receptor | 99.9 | 753 |
| 2 | NP_253244.1 | type 4 fimbrial biogenesis protein PilY1 | 99.6 | 1161 |
| 3 | NP_252857.1 | second ferric pyoverdine receptor FpvB | 99.1 | 802 |
| 4 | NP_249161.1 | ferrichrome receptor FiuA | 98.9 | 802 |
| 5 | NP_250468.1 | outer membrane porin F | 98.8 | 350 |
| 6 | NP_251378.1 | ferric enterobactin receptor | 98.7 | 746 |
| 7 | NP_253191.1 | glycine-glutamate dipeptide porin OpdP | 98.5 | 484 |
| 8 | NP_249979.1 | hypothetical protein PA1288 | 98 | 424 |
| 9 | NP_251970.1 | pyrophosphate-specific outer membrane porin OprO | 98 | 438 |
| 10 | NP_248982.1 | anaerobically-induced outer membrane porin OprE | 98 | 460 |
Table 4. Antigenicity score analysis for OMP candidates among 58 clinical isolate strains of PA. NF: not found.
| iron transport outer membrane receptor | type 4 fimbrial biogenesis protein PilY1 | second ferric pyoverdine receptor FpvB | ferrichrome receptor FiuA | outer membrane porin F | ferric enterobactin receptor | glycine-glutamate dipeptide porin OpdP | hypothetical protein PA1288 | pyrophosphate-specific outer membrane porin OprO | anaerobically-induced outer membrane porin OprE | |||
| 1 | PAO1 | 99.9 | 99.6 | 99.1 | 98.9 | 98.8 | 98.7 | 98.5 | 98 | 98 | 98 | |
| 2 | 97 | 99.8 | 99.4 | 98.9 | 99.4 | 98.8 | 98.8 | 98.4 | 94.1 | 98 | 98 | |
| 3 | AES1M | 99.9 | 99.4 | 99.1 | 98.9 | 98.8 | 98.9 | 98.2 | 94.1 | 98 | 98 | |
| 4 | AES1R | 99.9 | 99.4 | 99.1 | 98.9 | 98.8 | 98.9 | 98.2 | 94.1 | 98 | 98 | |
| 5 | ATCC 27853 | 99.9 | 99.4 | NF | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 6 | AZPAE15042 | 99.7 | NF | 99.4 | 99.4 | 98.8 | 98.9 | 98.3 | 98.2 | 97.8 | 99.1 | |
| 7 | CF39S | 99.8 | 99.4 | 99.5 | 99.4 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 8 | CI27 | 99.9 | 97.4 | 99.1 | 99.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 9 | DK2 | 99.9 | 97.4 | _ NF | 98.5 | 98.8 | 98.8 | 98.2 | 97.5 | 98 | 98 | |
| 10 | E6130952 | 99.8 | 99.5 | 98.9 | 99.3 | 98.8 | 98.8 | 98.1 | 98 | 98 | 98.9 | |
| 11 | F5677 | 99.8 | 99.4 | 99.7 | 99.4 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98 | |
| 12 | F22031 | 99.8 | 99.4 | 98.9 | 99.4 | 98.8 | 98.8 | 94.8 | 94.1 | 98 | 98.7 | |
| 13 | F23197 | 99.8 | 99.4 | 99.7 | 99.7 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98.7 | |
| 14 | F63912 | 99.8 | 97.5 | 99.1 | 99.1 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98.7 | |
| 15 | FRD1 | 99.8 | 99.4 | 98.4 | 99.3 | 98.8 | 98.8 | 98.5 | 98 | 98 | 98.7 | |
| 16 | H5708 | 99.9 | 99.4 | 98.7 | 99.3 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98.5 | |
| 17 | H27930 | 99.8 | 99.4 | 99.5 | 99.4 | 98.8 | 99.2 | 98.2 | 98 | 98 | 98.7 | |
| 18 | isolate F9670 | 99.9 | 99.4 | 99.7 | 99.7 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 90.9 | |
| 19 | isolate F30658 | 99.8 | NF | 99.7 | 99.3 | 98.8 | 98.9 | 98.5 | 98 | 98 | 98.7 | |
| 20 | isolate H47921 | 99.8 | 99.4 | 99.1 | 98.9 | 98.8 | 98.6 | 98.2 | 98 | 98 | 98.9 | |
| 21 | isolate M37351 | 99.9 | 97.4 | 99.1 | 99.4 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98.9 | |
| 22 | isolate T52373 | 99.9 | 97.3 | 99.5 | 99.5 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98.7 | |
| 23 | isolate T63266 | 99.7 | 99.4 | 99.7 | 99.2 | 98.8 | 98.9 | 94.1 | 98 | 98 | ||
| 24 | LES431 | 99.8 | 99.4 | 99.1 | 99.2 | 98.8 | 99.2 | 98.2 | 94.1 | 98 | 98 | |
| 25 | M1608 | 99.9 | 97.4 | 99.1 | 99.4 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98 | |
| 26 | MRSN12280 | 99.8 | 99.5 | 98.9 | 99.3 | 98.8 | 98.8 | 98.1 | 98 | 98 | 98 | |
| 27 | NCGM257 | 99.8 | 97.5 | 99.1 | 99.3 | 98.8 | 98.8 | 98.2 | 98 | 98 | 98 | |
| 28 | PA_D1 | 99.9 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 29 | PA_D2 | 99.9 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 30 | PA_D5 | 99.9 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 31 | PA_D9 | 99.9 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 32 | PA_D16 | 99.9 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 33 | PA_D21 | 99.9 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 34 | PA_D22 | 99.9 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 35 | PA1 | 99.8 | NF | 98.9 | 98.9 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 36 | PA34 | 99.9 | 97.5 | 98.9 | 98.7 | 98.8 | 98.8 | 98 | 94.1 | 98 | 98 | |
| 37 | Pa58 | 99.9 | 98.6 | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98.1 | |
| 38 | Pa84 | 99.9 | 98.6 | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98.1 | 98.1 | |
| 39 | Pa124 | 99.9 | 99.7 | 99.1 | 99.3 | 98.8 | 98.8 | 98.2 | 93.9 | 98 | 97.9 | |
| 40 | Pa127 | 99.9 | 99.7 | 99.1 | 99.3 | 98.8 | 98.8 | 98.2 | 93.9 | 98 | 97.9 | |
| 41 | Pa1207 | 99.9 | 99.4 | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 42 | Pa1242 | 99.9 | 97.9 | 90.9 | 98.9 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
| 43 | PA121617 | 99.8 | 99.4 | 99.5 | 99.4 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 44 | PACS2 | 99.9 | 99.4 | 98.8 | 99.4 | 98.8 | 98.9 | 98.4 | 94 | 98 | 97.9 | |
| 45 | RP73 | 99.8 | NF | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 46 | S86968 | 99.9 | 99.4 | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 47 | SCV20265 | 99.8 | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | ||
| 48 | T38079 | 99.9 | 99.4 | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 49 | UCBPP-PA14 | 99.9 | 97.4 | 99.1 | 99.4 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 50 | VA-134 | 99.8 | 99.4 | 98.9 | 98.5 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 51 | W16407 | 99.9 | 99.4 | 98.7 | 98.9 | 98.8 | 99.1 | 98.5 | 94.1 | 98 | 98 | |
| 52 | W36662 | 99.8 | 99.4 | 98.9 | 98.9 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 53 | W45909 | 99.9 | 99.4 | 99.1 | 98.9 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 54 | W60856 | 99.8 | 99.4 | 99.1 | 98.5 | 98.8 | 98.8 | 98.2 | 94 | 98 | 98 | |
| 55 | X78812 | 99.8 | 99.4 | 98.9 | 98.9 | 98.8 | 98.9 | 98.2 | 94.1 | 98 | 98 | |
| 56 | Y31 | 99.8 | 99.7 | 98.9 | 99.5 | 98.8 | 98.7 | 98.2 | 94.1 | 98 | 98 | |
| 57 | Y82 | 99.8 | 99.4 | 99.1 | 99.3 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 98 | |
| 58 | Y89 | 99.9 | 99.4 | 98.9 | 98.9 | 98.8 | 98.8 | 98.2 | 94.1 | 98 | 97.9 | |
Since antigens with homology to host proteins are likely to induce autoimmunity or immune tolerance, they must be eliminated from vaccine candidates. Vaxign2 uses BLAST for sequence comparison. In this study, we only selected proteins with no homology to human proteins (Table 2). Because proteins with a high number of helices can anchor on the surface of the bacterial cell, they may be out of reach of the host's immune system. Furthermore, multiple transmembrane domains make the purification of recombinant proteins difficult. Therefore, proteins with less than or equal to one transmembrane domain are considered more suitable in recombinant vaccine design. Hence, proteins with more than 1 transmembrane helix were excluded from this study (Table 2). CLC's main workbench was used to determine the physicochemical characteristics of the candidate vaccine. The molecular weight of candidate vaccine proteins is a significant factor in the recombinant production process. Usually, proteins with molecular weights (MW) ≤110 kDa are considered suitable candidates for recombinant production and purification (21). The only candidate with considerable molecular weight was type 4 fimbrial biogenesis protein (PilY1), with a MW of 126.582 kDa. The isoelectric point of the vaccine candidate proteins was predicted from 5.26 to 8.75, which indicates the acidic to alkaline nature. In our study, the protein stability index in a wide temperature range, i.e., alpha index, was reported as 66.1 to 72.96 for this vaccine candidate. All these parameters show the thermally stable nature of candidate vaccine proteins. The half-life of each vaccine candidate protein was predicted. The estimated half-life in mammals in laboratory conditions was predicted to be 30 hours and more than 10 hours in Escherichia coli. GRAVY index was -0.263 to -0.594, and the negative index indicates the hydrophilic structure of the vaccine, so it can interact well with water molecules. The molecular weight and other significant physicochemical properties of proteins are shown in Table 5. A vaccine based on conserved epitopes will likely remain effective against emerging variants because mutations are unlikely to occur in conserved regions. As the next step, 10 candidates were subjected to analysis by the InterPro-EMBL-EBI database and the presence of conserved domains was predicted only in 2 out of 10 proteins: outer membrane porin F (PS01068) and ferric enterobactin receptor (PS01156) (Table 6).
Table 5. Physicochemical properties of candidate proteins
| # | Protein Accession | Protein Name | Weight | Isoelectric point | Instability index | half-life mammals | half-life in E. coli | Aliphatic index | Grand average of hydropathicity (GRAVY) |
| 1 | NP_253204.1 | iron transport outer membrane receptor | 82336.61 | 5.72 | 22.18 | 30 hours | > 10 hours | 66.1 | -0.594 |
| 2 | NP_253244.1 | type 4 fimbrial biogenesis protein PilY1 | 126583.87 | 6.0 | 29.21 | 30 hours | > 10 hours | 67.05 | -0.500 |
| 3 | NP_252857.1 | second ferric pyoverdine receptor FpvB | 87431.25 | 5.60 | 27.31 | 30 hours | > 10 hours | 71.96 | -0.455 |
| 4 | NP_249161.1 | ferrichrome receptor FiuA | 88212.77 | 5.46 | 34.78 | 30 hours | > 10 hours | 72.51 | -0.480 |
| 5 | NP_250468.1 | outer membrane porin F | 37639.58 | 4.98 | 26.16 | 30 hours | > 10 hours | 69.94 | -0.443 |
| 6 | NP_251378.1 | ferric enterobactin receptor | 80967.53 | 5.65 | 36.81 | 30 hours | > 10 hours | 74.18 | -0.557 |
| 7 | NP_253191.1 | glycine-glutamate dipeptide porin OpdP | 53031.63 | 5.61 | 24.39 | 30 hours | > 10 hours | 70 | -0.484 |
| 8 | NP_249979.1 | hypothetical protein PA1288 | 45561.73 | 5.73 | 18.92 | 30 hours | > 10 hours | 78.231 | -0.263 |
| 9 | NP_251970.1 | pyrophosphate-specific outer membrane porin OprO | 47787.63 | 5.17 | 17.64 | 30 hours | > 10 hours | 64.703 | -0.499 |
| 10 | NP_248982.1 | anaerobically-induced outer membrane porin OprE | 49667.00 | 8.67 | 29.95 | 30 hours | > 10 hours | 72.96 | -0.436 |
Table 6. List of conserved peptides with their physicochemical properties
| Protein Accession | Protein Name | Weight | Isoelectric point | Conserved Intrepro domains | |
| 1 | NP_250468.1 | outer membrane porin F | 37.639 kDa | 5.26 | PS01068 |
| 2 | NP_251378.1 | ferric enterobactin receptor | 80.967 kDa | 5.89 | PS01156 |
In this article, we analyzed the genome information of 58 P. aeruginosa clinical isolates to introduce suitable vaccine candidates among OMPs. Based on our results, we suggested 10 candidate proteins that showed suitable characteristics, including OprF and ferric enterobactin receptors (Table 3). The increasing acquisition of broad-spectrum antimicrobial resistance genes leads to multiple drug resistance (MDR) phenotypes. It raises the treatment of PA infection as a challenging health problem globally (22). Therefore, and mainly due to the lack of efficient antibiotics, finding new intervention strategies is of grave importance. In this context, infection prevention by effective vaccines is considered a viable strategy (3, 23). Despite efforts starting from the 1970s, currently, there are no approved vaccines against PA, highlighting the necessity of developing secure and impressive vaccines (12). Currently, designing vaccines that contain minimal components from microorganism origin is trending. Such designs usually contain multiple antigenic epitopes from the same or several different pathogens and are known as recombinant multi-epitope vaccines. Recombinant multi-epitope vaccines are mostly peptide-based (24, 25). Hence, the introduction of peptides and proteins that have desirable antigenic characteristics is the first step in multi-epitope vaccine design. About 25% of the bacterial proteome comprises membrane proteins, approximately 2–3% of which are OMPs. In addition to their important role in transporting a broad range of molecules, including metal complexes, OMPs are also involved in bacterial pathogenesis and antibiotic resistance (26).
Characteristics of a valuable antigen include regions of structural consistency and chemical intricacy within the molecule, structural elements sufficiently different from the host, the ability to process the antigen by the immune system, and available immunogenic regions for antibody formation (27, 28). Therefore, since OMPs are positioned on the surface of bacteria, they are readily accessible to the immune system (29). For this reason, PA OMPs were our main target for investigation in this study. In addition to P. aeruginosa, the outer membrane proteins of other bacteria have also been considered as vaccine candidates. In 2017, Zhaohui Ni et al analyzed 33 complete genomes of Acinetobacter baumannii and 84 antibiotic resistance determinants using the Vaxign reverse vaccinology approach. They predicted classical-type vaccine candidates against Acinetobacter baumannii infections and new-type vaccine candidates against antibiotic resistance (16).
Among the PA vaccines that are in different stages of development, OprF-OprI systemic formulation (IC43) entered phase III clinical trials in 2020 (12). In accordance with the findings of Irum et al. (21), our results show that OprF had an antigenicity score of 98.8 among all 58 strains. OprF is a porin and forms small water-filled channels. Also, this protein plays a role in determining cell shape and can grow in a low osmolarity medium. Based on our results, OprF is one of the 10 nominated candidates. Although the low molecular weight of this protein can make its recombinant production and purification challenging, the presence of the conserved domain, PS01068, makes OprF stand out. In addition, as mentioned earlier, the possibility of producing multi-epitope vaccines provides the opportunity to engineer molecular weight and other physicochemical characteristics in the optimal range. PS01068 is found in the C-terminal part of proteins such as outer membrane protein ompA, a porin-like integral membrane protein from enterobacteria, Haemophilus influenza outer membrane protein P5, and Outer membrane protein P.III/class IV from Neisseria. It is worth mentioning that apart from this domain, these proteins are not structurally relevant. The OmpA-like domain appears to be responsible for non-covalent interactions with peptidoglycan and adopts a β-α-β-α-β-β fold (30, 31).
According to our results, from analysis by the InterPro-EMBL-EBI database, the only other OMP that contained a conserved domain is the ferric enterobactin receptor. Ferric enterobactin receptor conserved domain; PS01156 is also found in TonB protein from Escherichia coli (30, 31). Ferric enterobactin receptor has not been considered as an antigen among PA vaccine candidates previously. Physicochemical properties of this protein, although not in the optimum range, seem more suitable as a vaccine candidate than OprF (Tables 3 and 5). Among 58 species, this protein had an antigenicity score in the range of 98.7 (PAO1) to 99.2 (LES431). Type 4 fimbrial biogenesis protein PilY1 has the highest molecular weight amongst the remaining candidates. However, in 12 of the examined strains, type 4 fimbrial biogenesis protein PilY1 was not found in the genomic analysis, which seems to be due to incomplete annotation of genome records. The antigenicity score of type 4 fimbrial biogenesis protein PilY1 was between 99.7 and 97.3 among PA stains. Type 4 fimbrial biogenesis protein PilY1 is involved in various cellular processes such as pilus assembly, twitching motility, adherence to host cells, and type IV pili (T4P) initial assembly (32). As far as we know, none of the proteins discussed in the following discussion have been candidates for vaccine design against P. aeruginosa. Pyrophosphate-specific outer membrane porin OprO; which is an anion-specific receptor, with a higher affinity for phosphate (especially polyphosphates) than chloride ions, was another vaccine candidate that had an antigenicity score of 98.1 to 97.8 amongst the examined strains (25, 33). PA4514 encodes PiuA (iron transport outer membrane receptor). piuA is a TonB-dependent ferric siderophore receptor in the outer membrane of this bacterium. piuA has been shown to be under the regulation of Fur. PiuA is an important gene for survival in an iron-deficient environment and is up-regulated during iron limitation (26, 34). Iron transport outer membrane receptor that had an antigenicity score of 99.9 to 99.7 among the examined strains. When pyoverdine binds to iron, the resulting free pyoverdine is taken up by cells mainly through the action of the primary pyoverdine receptor FpvA. FpvA is necessary for the optimal absorption of pyrudine, and the secondary receptor FpvB can partially compensate for the lack of FpvA (29). The second ferric pyrudine receptor FpvB had an antigenic score of 99.7 to 90.9. The fiuA gene encodes ferrichrome receptor A, which is involved in the iron acquisition process. FiuA gene has pleiotropic functions that affect P.aeruginosa biofilm development and virulence (35).
The antigenicity score of ferrichrome receptor FiuA is 99.7 to 98.5. The OprD family of Pseudomonas aeruginosa contains 19 members, some of which facilitate the uptake of specific compounds into the cell. The members of this family share about 46-57% similarity in amino acid sequence, which is unusual among porin molecules. OpdP is a member of this family and is a glycine-glutamate dipeptide porin (36). OpdP has an antigenicity score of 98.5 to 94.8. OprE is one of the outer membrane proteins of Pseudomonas aeruginosa, whose expression is induced under anaerobic conditions. Anaerobiosis induces the production of OprE at the transcriptional level. OprE of Pseudomonas aeruginosa is one of the outer membrane proteins that form a channel with very small pores. Until now, the physiological role of OprE is unknown because the deficiency in OprE in strain PAO1 does not affect phenotypes such as growth rate or sensitivity to different antibiotics (37, 38). The antigenicity score of anaerobically-induced outer membrane porin OprE is 99.1 to 90.9. In addition to bacterial pathogens, reverse vaccinology has also been investigated as an emerging vaccine development strategy in viruses such as COVID-19 and herpes virus. In 2013, Zuoshuang Xiang et al analyzed 52 herpesvirus genomes using Vaxign and identified UL26.5 as a promising vaccine target for HSV-1 (18, 39). While the process of producing a vaccine from the start of research to the use of an approved vaccine can be very time consuming and expensive, the technique of reverse vaccinology (RV) can reduce the time required to identify protective antigens from 5 to 15 years to 1 to 2 years. However, further in vitro and in vivo analyses are necessary to confirm the safety and immunoreactivity of these proteins. In addition, the reverse vaccinology approach may help to develop strategies to combat the important and global problem of antibiotic resistance and to develop vaccines to combat these important antibiotic-resistant pathogens. However, so far only a few bacterial pathogens have been investigated with this approach (40, 41).
In this study, we only examined outer membrane proteins instead of all proteins of Pseudomonas aeruginosa. We did not check all the resistant strains. At the same time, reverse vaccinology can be a cost-effective, time-saving, and accurate approach to vaccine design compared to conventional methods that can be implemented from available tools.
Using 58 complete PA genomes and 9982 outer membrane proteins, we used the Vaxign2 pipeline and other bioinformatics methods. We were able to identify 10 vaccine candidates for the development of vaccines against PA infections. All predicted vaccine candidates had high antigenicity scores. Predicted antigens have no homology with human proteins and have less than 2 transmembrane helices with high adhesin probabilities. We found 2 OMPs with conserved domains, including outer membrane porin F and ferric enterobactin receptor. To our knowledge, our study is the first to apply reverse vaccinology with a focus on OMP for systematically predicting vaccine candidates against PA. Conducting clinical studies on these introduced candidate proteins as well as studying these antigens in other vaccine production platforms such as mRNA vaccine and studying the vaccinology features of these antigens in combination with new drug delivery methods such as lipid nanoparticles (LNP) can be useful. These antigens can also be used to design diagnostic tools. Also this pipeline can be used for other pathogenic bacteria as well. However, despite the extensive current research and previous studies in the path of finding a vaccine with optimal immunity and safety, the great diversity in the selection of vaccine candidate proteins seems to be a big obstacle in this path. To overcome this problem, a screening strategy with an approach to Uniform bioinformatics is recommended by the research community to find a vaccine with the highest immunogenicity and biosafety.
We want to thank all members of the microbiology laboratory of Kermanshah University of Medical Sciences. This work has been funded by the Kermanshah University of Medical Sciences (KUMS) and approved by the KUMS Ethics Committee (IR.KUMS.AEC.1401.020) as a partial fulfillment of the requirement for the PhD degree in Medical Bacteriology.
Conflicts of Interest
The authors declared no competing interests.
Received: 2023/06/28 | Accepted: 2023/09/19 | ePublished: 2023/11/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





