BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2109-en.html


 , Seyyed Behnam Abdollahi Boraei2
, Seyyed Behnam Abdollahi Boraei2 

 , Reza Erfanian3
, Reza Erfanian3 

 , MohammadReza Firoozifar3
, MohammadReza Firoozifar3 

 , Farnaz Hosseinzadeh Otaghvari4
, Farnaz Hosseinzadeh Otaghvari4 

 , Sarvenaz Atashian5
, Sarvenaz Atashian5 

 , Mohammadsadegh Zabihidan6
, Mohammadsadegh Zabihidan6 


2- Biomaterials and Tissue Engineering Research Group, Department of Interdisciplinary Technologies, Breast Cancer Research Center, Motamed Cancer Institute, ACECR, Tehran, Iran ,
3- Otorhinolaryngology Research Center, Tehran University of Medical Sciences, Tehran, Iran
4- Department of Cell and Molecular Biology, Faculty of Basic Science, University of Maragheh, Maragheh, Iran
5- Biomedical Engineering Department, Science and Research Branch, Islamic Azad University, Tehran, Iran
6- ENT and Head and Neck Surgery Research Center and Department, Rasool Akram Hospital, school of medicine, Iran University of Medical Sciences, Tehran, Iran
Since the end of 2019, SARS-COV-2 (COVID-19) has spread all around the world and become a worldwide problem with a lot of deaths (1). The COVID-19 infection shows some symptoms similar to influenza (headache, cough, fever myalgia, etc.). Also, there are other symptoms (olfactory and taste dysfunction) that were proven to expand during the pandemic (2). In general, the method of diagnosis and differentiation of patients with COVID-19 is a real-time polymerase chain reaction (RT-PCR) test, which is a time-consuming test and should be performed a few days after the onset of COVID-19 symptoms. Because time to diagnosis is so important in a pandemic, it must be possible for the early detection of disease to isolate patients as soon as possible before transmitting the disease to others. Therefore, recognizing and quarantining asymptomatic patients is a crucial and important step in managing the pandemic (3).
From the beginning of the pandemic, there were self-reported olfactory and taste impairments, which can be considered an appropriate and timely prediction of the disease. Studies in this field have observed olfactory dysfunction during the COVID-19 pandemic (4, 5). In March 2020, olfactory and taste symptoms were considered one of the first signs of COVID-19 by the American Academy of Otolaryngology, which suggested preventive quarantine after observing olfactory impairment symptoms to reduce the rate of disease transmission (6). Another study in Italy found that olfactory dysfunction in COVID-19 was more common in younger patients and women. An article states that anosmia and dysgeusia were identified as one of the first symptoms of COVID-19 disease, but this study did not address the relationship between severity, mortality, and pathogenesis (7). How the virus affects the senses of smell and taste is still unknown. Significant advances in cellular and molecular mechanisms of virus effect on olfactory disorders have been made. Recent studies have provided new findings on olfactory epithelial cell types that express virus-associated proteins (8). It seems that ACE2 is expressed in the tongue’s epithelial cells, but perhaps not in the taste buds. Nowadays, ACE2 suppressor drugs are considered stimulants of olfactory and taste impairments (Figure 1) (9). Evidence suggests that there is a cascade of cellular events occurring in the olfactory epithelium that may explain the cause of olfactory dysfunction due to COVID-19.
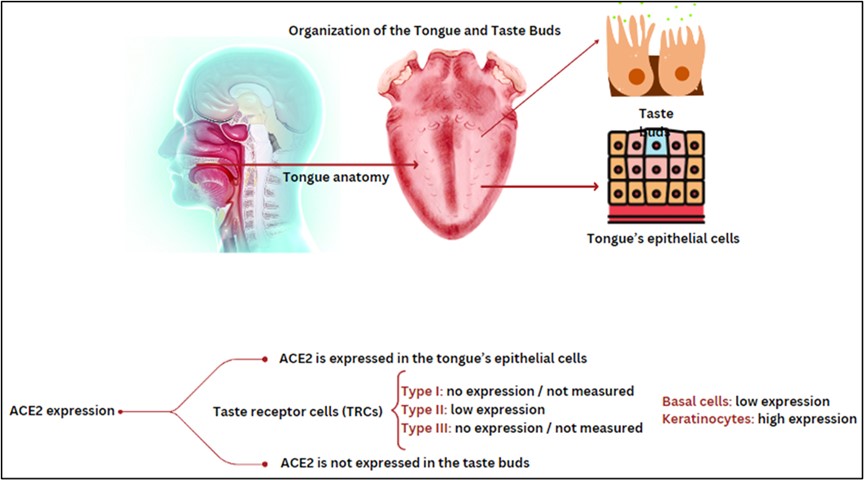
Figure 1. Shows the mechanisms of ACE2 expression in the tongue (Designed by the authors, 2024).
In this present research, we study a group of people infected by COVID-19 to specify the relationship between smell dysfunction and the disease. To understand this matter, we compare the results of the smell identification test with other diagnostic tests such as RT-PCR test, ESR, CRP, and lung CT scan. Finally, the results were analyzed to determine the ability of smell dysfunction as a quick, effective, and early marker of screening COVID-19 and the accuracy of ISIT in recognizing COVID-19 patients.
This is a cross-sectional study. 94 patients with COVID-19 who were admitted to the Amiralam hospital at the peak of the delta variant in Iran were confirmed to participate in this study. They had positive RT-PCR assay results for SARS-CoV-2 nucleic acids or a remarkable absorption of infection signs on their chest CT images. Regarding the demographic characteristics of the study population, the following can be mentioned: The age group of the study was from 18 to 83 including 53.2% male and 46.8% female. Smoking was common in 19.1% of the patients. 2.1% of the participants had an in-hospital job. Regarding the underlying diseases, the following can be mentioned: 23.4% had a history of high blood pressure, 12.8% had diabetes, and 4.3% had a history of heart disease. Nasal congestion and asthma each have occurred in 2.1% of the patients. There was no one with hypothyroidism, Alzheimer's, chronic bronchitis, and a history of head trauma. However, 12.8% of the participants had other underlying diseases.
2.1. Patient Self-reported Symptoms
The first reported symptoms by patients included body pain, fever, cough, olfactory dysfunction, decrease in taste, shortness of breath, headache, and other symptoms.
2.2. Clinical Tests
The initial examinations including body temperature, O2 saturation, and heart rate were carried out for the patients. Then the complete blood test was performed to monitor the amounts of ESR, CRP, Hg, Lymphocyte, Leucocyte, and Platelet. The olfactory function of the patients was assessed by the Iran Smell Identification Test (ISIT). ISIT is a 24-item smell identification test, for which subjects are asked to scratch and sniff the items and select one of the four alternatives (for example, “banana,” “mint,” “gasoline,” or “cinnamon”). The score of ISIT is determined by the number of correct answers of the subjects. So, it can be a number between zero to 24. A score ≥ 20 is considered a normal range and a score ≤19 is considered an olfactory disorder. The results of clinical tests were statistically analyzed to determine the most accurate and rapid test for COVID-19 detection (Figure 2). Exclusion criteria include people who are unable or unwilling to perform ISIT.
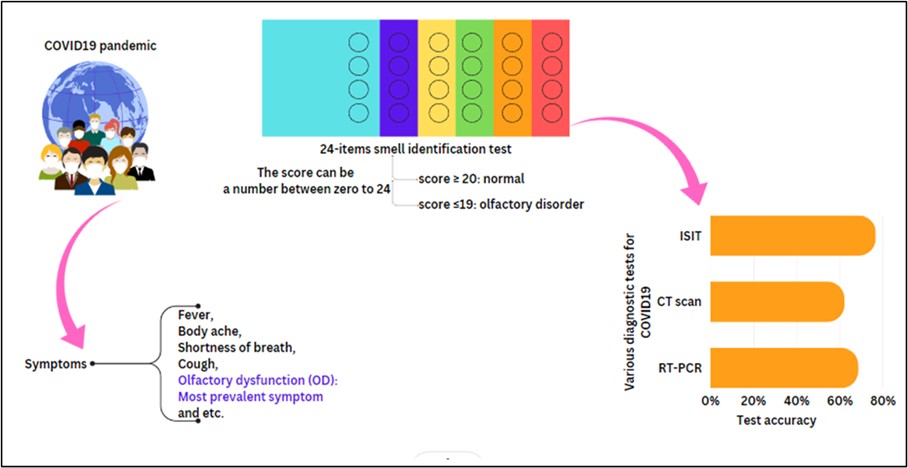
Figure 2. Schematic illustration of materials and methods of the study (Designed by the authors, 2024).
3.1. Patients Self-reported symptoms
Table. 1 shows the frequency of common symptoms based on the patient's self-reported. As it can be seen the olfactory dysfunction by 68.1% is the most common symptom in the patients with delta variant. Subsequently, body aches by 57.4%, a decrease in taste by 53.2%, and fever by 51.1% were more frequent than the other symptoms. Cough by 25.5%, shortness of breath by 19.1%, headache by 14.9%, and other symptoms like nausea, weakness, and fatigue at 6.4% are located at the bottom of the list.
Table 1. Patients self-reported symptoms
| Symptoms | Total | YES | Percent (%) of positive answers | NO | Percent (%) of negative answers |
| Olfactory dysfunction | 94 | 64 | 68/1 | 30 | 31/9 |
| Body pain | 94 | 54 | 57/4 | 40 | 42/6 |
| Decrease in taste | 94 | 50 | 53/2 | 44 | 46/8 |
| Fever | 94 | 48 | 51/1 | 46 | 48/9 |
| Cough | 94 | 24 | 25/5 | 70 | 74/5 |
| Shortness of breath | 94 | 18 | 19/1 | 76 | 80/9 |
| Headache | 94 | 14 | 14/9 | 80 | 85/1 |
| Other Symptoms | 94 | 6 | 6/4 | 88 | 93/6 |
3.2. Clinical Tests
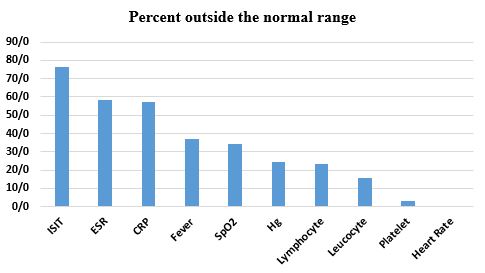
As mentioned above, full clinical tests and examinations were performed for the patients. Then their olfactory function was assessed by the ISIT. The results of the tests are displayed in Table 2. According to these results, 76.6% of the patients have an olfactory disorder which was detected by ISIT. It means that they obtained a score less than 19 on the smell test. 58.5% of the patients show Erythrocyte Sedimentation Rate (ESR) results outside the normal range indicating that an inflammatory condition may be present. C-reactive protein (CRP) a type of protein that is associated with inflammation in the body shows increasing measures in 57.4% of the patients. 37.2% of the patients were found to have a fever and 34% presented SpO2 less than 95. Results of the blood test also show that the hemoglobin level for 24.5% of the patients was outside the normal range. A rise in the lymphocyte and leucocyte count was observed in 23.4% and 16% of the participants respectively. Patients with platelets outside the normal range are less than 4%. Nobody did not have an abnormal heart rate. Figure 3. Shows the percent (%) outside the normal range for the results of the clinical tests.
Table 2. Results of the clinical tests
| Parameters | Total | Average | Standard Deviation | Min | Max | Normal range | Numbers outside the normal range | Percent outside the normal range |
| ISIT | 94 | 15.29 | 5.190 | 3 | 24 | 0-24 | 72 | 76/6% |
| ESR | 94 | 28.19 | 20.74 | 2.00 | 79.00 | female: <29 Male: <22 |
55 | 58/5% |
| CRP | 94 | 34.03 | 57.91 | 1.00 | 231.00 | <3 | 54 | 57/4% |
| Fever | 94 | 37.33 | 0.525 | 36.5 | 38.5 | 36.8-37.2 | 35 | 37/2% |
| SpO2 | 94 | 94.39 | 3.817 | 80 | 98 | 95-97% | 32 | 34/0% |
| Hg | 94 | 14.56 | 2.66 | 9.00 | 17.5 | female: 12-16 Male: 14-18 | 23 | 24/5% |
| Lymphocyte | 94 | 2.81 | 1.21 | 0.18 | 4.50 | 0.8-5 | 22 | 23/4% |
| Leucocyte | 94 | 9.96 | 13.89 | 0.42 | 91.00 | 5-10 | 15 | 16/0% |
| Platelet | 94 | 273.84 | 61.35 | 166.0 | 408.00 | 150-400 | 3 | 3/2% |
| Heart Rate | 94 | 84.56 | 7.74 | 72.00 | 100 | 60-100 | 0 | 0/0% |
Figure 3. Percent (%) outside the normal range for the results of the clinical tests (Designed by the authors, 2024).
3.3. Covid-19 specialized diagnostic tests
As mentioned above, the participants in this study carried out at least one of the Covid-19 specialized diagnostic tests including RT-PCR assay and chest CT scan. We checked their medical records and found out that an RT-PCR assay was performed for 73 patients and a chest CT scan was performed for 66 patients. 45 patients carried out both tests. Table 3 shows the results of COVID-19 specialized diagnostic tests. As can be observed, 68.5% of patients were found to have a positive RT-PCR assay and 62.1% of the patients had a remarkable absorption of infection signs on their chest CT images. A simple comparison of these results with the results of the olfactory test can be seen as the higher accuracy of the olfactory test for the early diagnosis of COVID-19.
Table. 3. Results of COVID-19 specialized diagnostic tests
| Tests | Total | The number of patients who have a diagnostic test result | Test Results | |||
| Number of Positive | Percent (%) of Positive | Number of Negative | Percent (%) of Negative | |||
| RT-PCR | 94 | 73 | 50 | 68/5 | 23 | 31/5 |
| Chest CT scan | 94 | 66 | 41 | 62/1 | 25 | 37/9 |
This study aimed to evaluate the prevalence of olfactory dysfunctions in COVID-19 patients by self-reported methods and by using the Iran Smell Identification Test (ISIT). We also evaluate the accuracy of ISIT for rapid screening of COVID-19 in comparison with existing diagnosis tests.
Olfactory dysfunction (OD) is one of the common symptoms of COVID-19, but it isn’t a novel phenomenon and there are a lot of viruses that can make OD, including influenza virus, parainfluenza virus, adenovirus, poliovirus, coxsackievirus, herpesvirus and enterovirus (11-13). The COVID-19 virus has been reported to enter the body through the olfactory epithelium at the roof of the nose (14). The task of detecting and transmitting odor information to the brain is performed by sensory neurons in the olfactory epithelium. It should also be noted that a special feature of the basal cells of the olfactory epithelium is that it can regenerate throughout life. It has also been reported that OD, in addition to damaging the olfactory epithelial nerve and extending to the olfactory bulb, may express ACE2 and TMPRSS2 proteins by stem cell neurons (15, 16). Because the expression of these proteins can vary from person to person, the duration of OD varies due to different degrees of damage to the olfactory neurons, and their repair may take several months (Figure 4) (17, 18). Due to the high prevalence of OD in COVID-19 disease, patients need to be consulted about the possibility of recovery. It should also be possible to identify people with a high probability of persistent OD so that appropriate treatment choices can be made. According to the data collected, about 33% of cases recover after a maximum of 14 days, and also 34% did not recover even after 45 days (19).
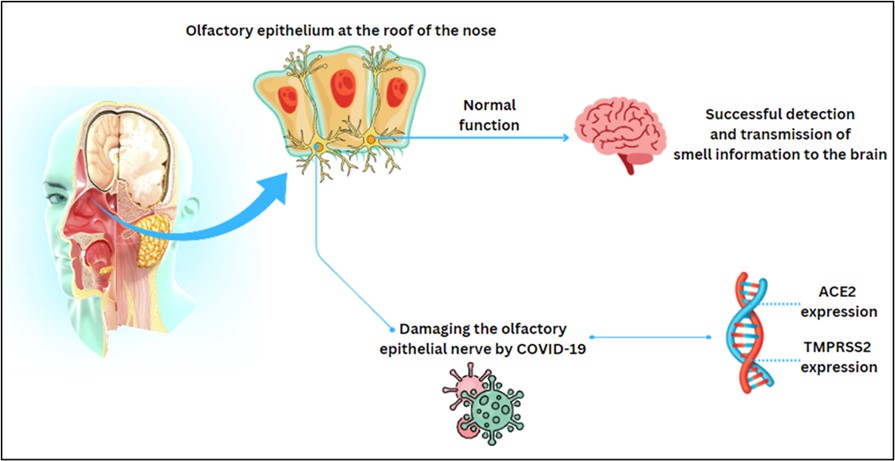
Figure 4. The normal function of the olfactory epithelium and the damaging procedure of olfactory epithelial nerves (Designed by the authors, 2024).
Public health screening for COVID-19 is becoming a routine activity. It has become usual for people to check their body temperature during the pandemic because fever is a key indicator of infection. Scientists, however, propose that taking a temperature is a weak indicator of COVID-19 in older adults and that a pulse oximeter be used instead. The reliability of a pulse oximeter as an indicator of COVID-19 has been the subject of debate in recent months. Recently, the World Health Organization (WHO) listed the use of the pulse oximeter to identify COVID-19 patients who may need to be hospitalized due to low oxygen levels. Based on this study, we find that the value of fever and oxygen saturation of blood are much lower than the olfactory disorders. Therefore, we propose using the Smell Identification Test as a rapid screening for COVID-19. ISIT is a noninvasive, rapid, and easy test and it can be applied for public health screening. ISIT also showed higher accuracy compared with the COVID-19 specialized diagnostic tests including RT-PCR assay and chest CT scan. However, further studies are needed to confirm these findings.
The strength of our study is that it is the method of data collection, the proven olfactory test by the Food and Drug Organization of the Ministry of Health of Iran, a suitable sample size, and the appropriate bio-statistical studies. Also, the weaknesses of the study include: first the sample size and second, the limitation of RT-PCR for the detection of COVID-19 virus, in nasopharyngeal swab samples, which has a less value than the standard, which leads to a high number of false negatives (20).
In summary, in this systematic review study, it was found that there is an acceptable association between lncRNA and SNP with tuberculosis and M. tuberculosis. Therefore, it was found that these regulatory factors play an important role as diagnostic biomarkers and the development of new therapies. However, more studies are needed to discover new lncRNAs and their association with tuberculosis.
Declared none.
Conflicts of Interest
All authors – none to declare.
None declared.
Received: 2023/08/22 | Accepted: 2023/11/18 | ePublished: 2024/03/18
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |