BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2093-en.html


 , Ali Mojtahedi2
, Ali Mojtahedi2 

 , Mohammad Faezi Ghasemi1
, Mohammad Faezi Ghasemi1 

 , Khosro Issazadeh1
, Khosro Issazadeh1 

 , Mostafa Golshekan3
, Mostafa Golshekan3 


2- Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran ,
3- Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Rasht, Iran
Pathogenic bacteria are essential objectives for diagnosis in different samples such as the pharmaceutical industry, general health, and food safety. Significant numbers of morbidity and mortality are a result of infectious diseases universally (1). Various foodborne pathogenic microorganisms such as bacteria, viruses, parasites, and fungi can have the potential to cause more than 250 known illnesses. In developing countries, about 1.7 billion children under the age of five die because of diarrhea due to drinking contaminated water. Furthermore, poor water quality and hygiene are the cause of 525000 children's deaths globally (2, 3). One of the important indicator bacteria in the pharmaceutical quality control and additional examples is E. coli (4). There are four conventional methods for E. coli diagnosis, e.g. plate count method, Multiple Tube Fermentation (MTF), Most Probable Number (MPN), and Membrane Filtration Technique (3, 5, 6). These methods need several steps, experienced staff, and a minimum time of 24-48 h to get sufficient results assurance (7). Recently, a number of biosensors and rapid tests have been designed to detect foodborne pathogens. Being sensitive and cost-effective to limit damages is essential for rapid identification test methods for pathogens (8, 9). Quantitative, qualitative, identification, validation, limit of detection, sensitivity, precision, and verification of rapid detections are very valuable (10). As an illustration of carbon-based materials, Garcia et al. created an electrode using single-walled carbon nanotubes (SWNTs) that were combined with specific antibodies (Ab) to detect whole cells or lysates of E. coli O157:H7. The detection limit for this method was 105 and 103 CFU (colony forming units) per mL (11).
Graphene nanocomposites can be used as electrochemical labels for immobilizing biomolecules on electrode surfaces to enhance amplifying detection signal (12).
There wasn't any experience in E. coli detection by biosensors in the pharmaceutical industry. This study is a new biosensor modification with rGO with immobilized Au NPs for E. coli detection.
rGO Synthesis
An improved Hummer technique was performed for the conversion of graphite powder to graphene oxide (GO). Under stirring conditions at room temperature, graphite powder (6 g) was added into a 500 mL conical flask including 100 mL of concerted H2SO4. Afterward, NaNO3 was added gradually, with stirring, until 3 grams had been added. The mixture was stirred for half an hour and then cooled to 0°C using an ice bath. The solution was vigorously stirred when 12 g of KMNO4 was gradually added and then agitated for 1 hour. The reaction's temperature was then raised to 35°C and stirred for an additional 1.5 hours. Next, 150 mL of deionized water was added and the solution was stirred at 98°C for 15 minutes. The achieved paste color is brown. The suspension cooled to 35°C during stirring and then deionized water (850 mL) was added. The bright yellow color of the media was achieved after adding 400 mL of H2O2 (10%). The resulting GO was isolated and washed with 500 mL of 1:10 HCl aqueous solution followed by 1.5 L deionized water to obtain pH 7. The GO gel was filtered and dehydrated at 60 °C for 12 h.
In this study, reduced graphene oxide (rGO) was produced according to the method described by Assari et al. To make the rGO, 300 mg of pre-prepared GO powder was dissolved in 300 mL of water and then subjected to ultrasonication for 1 hour. (13). Chemical molecules of GO and rGO were checked with the Infera Red technique.
Immobilization of Designed Glassy Carbon Electrode
The surface of the GCE was cleaned using a polishing cloth with a water slurry containing 0.3 and 0.05 μm alumina particles. Afterward, the GCE was sonicated in acetone for 3 minutes, washed with distilled water, and then left to dry at room temperature. (13).
a. Gold nanoparticles were immobilized on rGO with two methods:
1. Tetrachloroaurate trihydrate (HAuCl4. 3H2O) was exhausted for Au NPs synthesis with some reduction agents such as sodium borohydride and trisodium citrate. The flask containing 100 mL of an aqueous solution of chloroauric acid with a concentration of 0.01 wt% was being stirred vigorously when 1 mL of 1 wt% aqueous trisodium citrate solution with a concentration of 1 wt% was added to it. Next, 1 mL of a solution of NaBH4 in 1 wt% aqueous trisodium citrate with a concentration of 0.075 wt% was added, and the mixture was stirred for 5 minutes. The wine-red color of the solution represents Au NPs (13). They were dispersed in the liquid phase and it was impossible to separate.
2. To prepare the solution, 50 mg of HAuCl4. 3H2O was dissolved in 100 mL of PW and boiled for 15 minutes. After that, 100 mg of trisodium citrate was added to the solution and stirred for 5 minutes. Wine red of solution color demonstrating particle construction. After evaporation of water, HCl 10% was added for the cleanness of Au nanoparticles, then they washed and adjusted pH to 7 and dried at 50°C overnight.
Suspensions (1:1) of produced gold nanoparticles and rGO in ethanol were prepared separately and sonicated for 3 hours. They were strung for 1 h. 10 μL of the uniform suspension of Au-rGO dropped and dried at 45°C for 30 min.
b. To prepare the electrode, a 5 μL solution of rGO (1:1) in ethanol was uniformly applied onto the surface of a glassy carbon electrode and allowed to dry in the air. For the chronoamperometric technique, a potential of −0.2 V was applied for 250 s, which resulted in the electrodeposition of Au NPs onto the surface of GCE/rGO. This electrodeposition was carried out in a deaerated 0.5 M H2SO4 solution containing 0.3 mM HAuCl4. The electrode was then washed with deionized water and dried at 33°C. HAuCl4 was made from Au with HNO3: HCL (1:1).
Two modified electrodes, GCE/rGO-Au, and GCE/rGO/Au NPs, were subjected to a combination of 5 mM N-hydroxysuccinimide (NHS) and 2 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochlori- de (EDC). This reaction took place at 33°C for 2 hours while the solution was stirred slowly to activate the groups present on the surface. Next, the modified electrodes, GCE/rGO-Au NPs or GCE/rGO/Au NPs, were treated with 5 μl/10 mL of E. coli polyclonal antibodies (in 0.1 M PBS, pH 7.4) for 1 hour at 33°C, after which the electrodes were rinsed with 0.1 M PBS (pH 7.4) and allowed to dry for 30 minutes at 33°C. To prevent any unoccupied sites on the GCE/rGO/Au NPs/E. coli polyclonal antibody complex, a 5 μl solution of 0.5 W/V% BSA was applied to the modified electrode and allowed to dry at 33°C for 30 minutes. In this study, we used freshly designed electrodes for each section.
Electrochemical Measurements
The modified electrodes were subjected to morphological and elemental characterizations using SEM. The electrochemical investigation of E. coli and a comparison of currents in different stages of modification were carried out using a three-electrode system consisting of a reference electrode, a platinum wire, and a GCE. The CV technique, SWV, and DPV methods were used in a potential range of 0 to +1.3 V at a scan rate of approximately 100 mV s−1 to investigate the performance of the modified electrodes, and the best modification was chosen. After all, several dilutions of E. coli (ranging from 1×101 to 1×108 CFU/mL) inoculated in 0.1 M PBS with 0.5 mM sterile acetaminophen were applied for electrochemical measurements. Acetaminophen was an indicator molecule that had no side effects on bacteria.
Preparation of Test Strains
The bacterial strains comprising E. coli ATCC 8739 were obtained. To cultivate E. coli from a lyophilized vial, Tryptic Soya Agar (TSA) was used as the culture medium and incubated at 37°C for 24 hours. Bacterial cell counts were determined using the streaking method. The bacteria were then transferred to Tryptic Soya Broth (TSB) and centrifuged at 15000 × g for 15 minutes. The resulting bacterial solution was diluted in 0.1 M PBS (pH 7), and viable bacterial cells were counted using the plate count method on TSA. Additionally, the bacterial solution was subjected to serial dilutions, and the OD 600 nm was measured using a spectrophotometer.
Microbial Sample Preparation
To identify E. coli using the classical method, various dilutions of the bacteria (ranging from 1×101 to 1×108 CFU/mL) were inoculated into 0.1 M PBS (pH 7.4) containing 0.5 mM acetaminophen and 0.1 M PBS (pH 7.4). The pour plate method was used to count the viable bacterial cells. Biosensor application was investigated using the SWV technique. Additionally, various dilutions of E. coli (ranging from 1×101 to 1×108 CFU/mL) were prepared in 100 mL of Soybean-Casein Digest medium and incubated at 30–35°C for 18 to 24 hours. One ml of each culture was then transferred to 100 ml of Mac Conkey and incubated at 42–44°C for 48 hours. Consequently, the enriched media was subjected to sub-culturing on Mac Conkey agar and Eosin-Methylene Blue Agar (EMB) media plates and then incubated at 30-35°C for 18 to 48 hours following the USP and PB guidelines. (5, 6). The effectiveness of the total count method product was validated by adding various dilutions of E. coli (ranging from 1×101 to 1×108 CFU/mL) to a diluted sample (diluted at a ratio of 1:10) in 0.1 M PBS (pH 7.4) containing 0.5 mM acetaminophen.
Specificity, interference, repeatability, and sustainability
To determine the specificity of the immobilized electrode compared to Escherichia coli ATCC 8739, the SWV method was tested using a dilution of 1×105 CFU/mL of each reference strain, including Salmonella enterica subsp. enterica serotype Typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 9027, Bacillus spizizenii ATCC 6633, and Staphylococcus aureus ATCC 6538. Also, an investigation was carried out to determine the interference of uric acid with acetaminophen using a concentration of 100 µM. Four groups of tests were conducted in 0.1 M PBS, sterile 0.1 M PBS with 0.5 mM acetaminophen, 100 µM uric acid in 0.1 M PBS, and 100 µM uric acid in 0.1 M PBS with 0.5 mM acetaminophen. These groups were chosen to determine the potential interferences of the bare and modified electrode using the SWV method (E1 = 0, E2 = 1.3, scan rate: 100 mV.s-1).
Research Studies
For data analysis in this study, the mean ± standard deviation (SD) of three separate experiments conducted in triplicate was used.
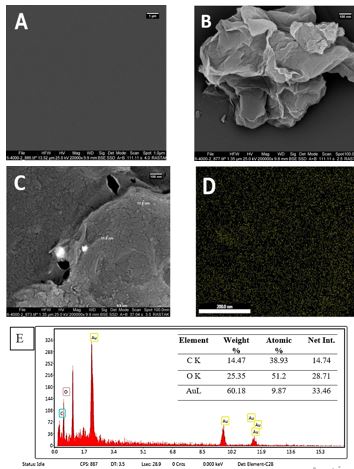
Identification of the situation and structure of GCE, rGO, and Au NPs scattered on GCE were confirmed with SEM (Figure 1).
Figure 1. (A) Bare GCE surface, (B) surface of rGO deposited on GCE, (C) Au NPs on rGO /GCE surface (D) total map of Au NPs decorated on rGO /GCE surface (E) EDX of GCE/rGO/Au NPs
Energy dispersive X-ray spectroscopy was used to examine the composition and distribution of elements in the composite of GCE/rGO/Au NPs. (Figure 1E). 60.18 weight percent and the purity of Au NPs in rGO modification were established with EDX scrutiny.
Immunosensor features
After the immobilization of rGO, the methods used for the bare GCE showed an increase in the displayed current, with an approximate fixed peak. The modification with rGO increased the effective surface area of the electrode.
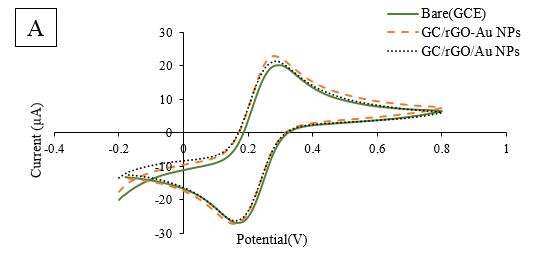
Two different methods of Au NPs modification established that the chloramperometric method had more increase current increase (Figure 2A). Also, GCE/rGO/Au NPs responses didn't have a sufficient increase in comparison with GCE/rGO (Figure 2B, C, D).
The immersion of antibodies on the surface of the modified electrode resulted in a decrease in the related current. This is due to the reduction in active sites for electron transfer and the barriers generated by polyclonal antibody immobilization. To mitigate this, the surface of the modified electrode was blocked with BSA. However, the height of the current peak declined due to the protein coating on the electrode's surface. As a result of binding BSA to the electrode surface, the SWV curve showed a weaker current peak. Additionally, during this reaction, there was a slight increase in current.
Figure 2A. Comparative Cyclic Voltammetry (CV) of two methods of Au NPs modification in GCE/rGO
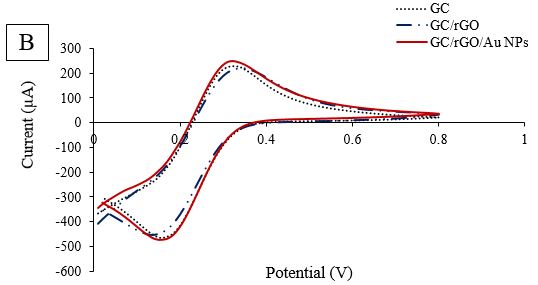
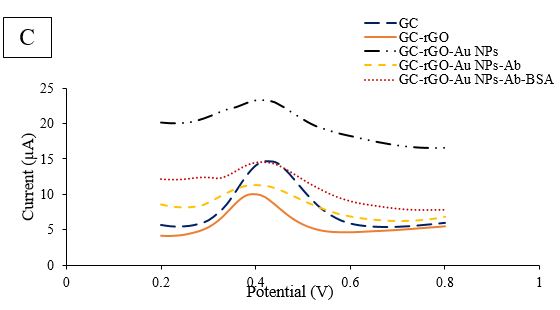
Figure 2B. Comparative CV of GCE/rGO/Au NPs Modification
Figure 2C. Comparative DPV of GCE/rGO/Au NPs/Ab/BSA Modification in scan rate 100 mV.s−1
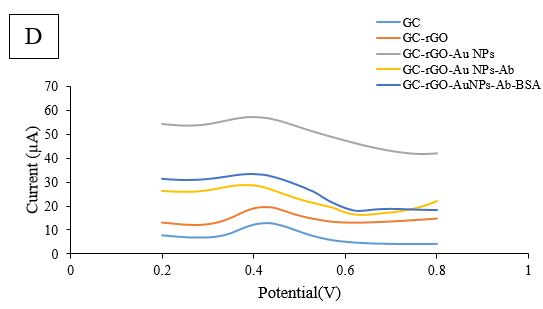
Figure 2D. Square-wave voltammetry (SWV) of GCE/rGO/Au NPs/Ab/BSA Modification in scan rate 100 mV.s−1
To further evaluate each stage of modification, additional findings were obtained. The CV technique was used as an amperometric detection method, with 5mM K3Fe(CN)6 in 0.1M KCl solution at -0.2 to 0.8 V, and a scan rate of 100 mV.s-1 was employed (Figure 2B). When comparing the oxidation and reduction peaks obtained from the bare electrode, there was a slight increase in current after rGO coating. However, after Au NPs were exposed to rGO, no noticeable current enhancement was observed. Furthermore, the decreasing trend continued during the third stage with the immersion of the electrode in the antibody. In the last stage of modification, when BSA solution was dropped onto the designed electrode surface, the peaks had a significant reduction compared to GCE/rGO/Au NPs/E. coli Abs. The coating of BSA protein molecules on the electrode surface led to a decrease in current. To further explore the designed electrode, another investigation technique, DPV, was performed at a potential rate of 0-0.8V. The results of the DPV investigation were comparable with the detected SWV results (Fig. 2D). After rGO deposition, the height of the peak slightly increased. Despite Au NPs immobilization, the current diminished, as shown in Fig. 2C, and the trend of reduction continued in the presence of antibody and BSA molecules.
Estimation of Electrode Surface Area and Optimization of Conditions
As a result of unapprovable conditions of peaks during the immobilization, estimation of electrode surface area and optimization of conditions couldn't be performed. When using the SWV technique for scanning, a noticeable peak was detected at 0.4 volts potential in the presence of acetaminophen and various E. coli populations. The peak had a detectable height of the peak. To investigate the newly designed immunosensor, the SWV peak current (from 0 to 0.7 V) and E. coli populations (ranging from 1×101 to 1 × 108 CFU/mL) were utilized.
Repeatability, Reproducibility, Specificity, and Enforcement
The repeatability factor can be demonstrated by the similarity between continual measurements of the same sample accomplished at least three times using one immobilized electrode. On the other hand, the indenture between signals achieved with the same technique under different immobilization conditions represents reproducibility. However, the process of repeatability for the newly designed immunosensor was canceled due to the lack of acceptable data during detection.
Current Methods and Designed Biosensors for E. coli Detection
No negative effects of acetaminophen molecules were observed in E. coli detection during the investigation of two test groups (with and without acetaminophen). The pour plate technique and rapid detection of E. coli were carried out using the newly designed biosensor. According to observed results Acetaminophen molecule had no prevention effect on the population of bacteria and we obtained very similar data between the two mentioned groups by conventional test method (P<0.05) and no inhibitory factor on E. coli growth was observed. For comparison of E. coli detection in the range of (1×101– 1×108 CFU/mL) with the acetaminophen group by a classical method was performed but biosensor detection wasn't performed because there weren't acceptable results with this modification.
According to USP44 and BP2022, there are classical methods for E. coli detection in water, raw materials, and finished products (5, 6). Classical methods have a lot of challenges (14). Various interpretations, inheritance of long test time, low sensitive methods, validations, suitability, and time-consuming methods are noticeable problems (15, 16). The classical methods have a recovery ability of all present microorganisms in the samples. Other factors that can cause interference are the conditions during incubation and the problem of microorganisms that are viable but non-culturable (17).
In 2020, Meraat et al. made a biosensor based on ZnO NPs and MWCNTs that could be applied to the β‑Galactosidase activity of E. coli with a detection limit of 101 CFU/mL in 15 min (18). The amount of current signal was improved in graphene-E. coli/GCE by DPV technique investigation (19). Lipopolysaccharide biomolecule in E. coli was detected with a highly selective method with GCE/rGO/Au NPs (20).
In 2020, a new design of electrode, IgG/rGO/SPCEs (screen-printed carbon electrode), was utilized for detecting E. coli in water (21). In 2020, Yang and colleagues utilized a CIP@MWCNT-based aptasensor to detect 3.15×102 CFU/mL of E. coli O157:H7 in contaminated milk after pre-incubation for one hour (22). In 2020, Yang and colleagues modified a label-free impedimetric biosensor for detecting E. coli by utilizing a polyaniline (PANI) and graphene (G) composite on a GCE (16). rGO modified with M13 phage with LOD 45 CFU/mL tested by Nakama et al. (23). Au NPs/rGO–PVA/GCE modified by Qaanei M. for E. coli detection in some samples from real-world scenarios such as tap water, milk, and meat samples with LOD 9.34 CFU mL−1 (24).
Silva et al. designed a nanocomposite based on rGO and gold nanoparticles on GCE applied in the detection of sulfamethazine (SMZ) in swine (25) and it wasn't used for bacterial detection.
GCE modification had shown that rGO couldn't be practical for increasing current. In the electrochemical immunosensing strategy, Au NPs are very applicable in immobilization because they increase conductivity, affinity, biocompatibility, and electroactivity. Obtaining a certain size and shape of AuNPs by the chemical synthesis procedures is a great challenge in modification (26). In this study, we tried to use two methods for Au NPs modification with different efficiencies. A stabilizing agent such as citrate has good potential for reduced nanoparticles, so their sizes will develop to at least 30-300 nm. Another effect of Au NPs is the increase of the active electrode surface. Further Au NPs effect in modification is capturing antibodies that can easily bind with target antigen in higher concentrations to give the lower detection limit (27). In this study, because of more concentration and size of Au Nps, the chloramprometric method was applied for decoration. Despite several methods for Au NPs immobilization, increasing currents was not significant. Even if amperometry gold nanoparticle was not successful for increasing severance of current during immobilization of electrode with rGO decoration. Acetaminophen molecule had qualified current reactions to E. coli concentrations in samples without any negative effect on growth bacteria. 0.1M PBS (pH 7.4) was a good choice for dilutions. The CV technique was not highly selective for the test, as it had to be performed in a mixture of 2 mM PBS (pH 7.0), 5.0 mM K3[Fe(CN)6]/K4[Fe(CN)6] (1:1), and 0.1 M KCl, with a potential range of -0.5 to +0.7 V (vs. Ag/AgCl) (28). The toxic effects of CN elements can harm actual results in viable bacteria. The use of polyclonal antibodies was a suitable option for detecting all strains of E. coli in different types of samples.
In brief, GCE/rGO/Au NPs/E. coli PAbs/BSA biosensor was not an achievement. It was not feasible to use it for the rapid and accurate detection of all species of E. coli strains with small sample volumes. It would be a good suggestion to use another design of biosensor with another kind of carbon nanotubes for E. coli detection in pharmaceutical products, water, and foods or real samples with a passing validation process.
The authors have confirmed that all ethical standards required for preparation and publication of the manuscript have been followed. The manuscript has not been published elsewhere, nor is it currently under consideration for publication in any other journal.
The authors declare that data and related material have not been published elsewhere nor is it under consideration in any other journal.
Not applicable.
Ali Mojtahedi and Mohammad Faezi Ghasemi Supervised, Conceptualized, and the data investigated, Khosro Issazadeh also reviewed the data, and Mostafa Golshekan helped with designing the biosensor and the analytical parts of the study. Fatemeh Behoftadeh did the experiments throughout the study as project administration and wrote the original draft.
This research was conducted as part of a Ph.D. thesis by Fatemeh Behoftadeh. The laboratory facilities for this study were provided by Alborz Darou Pharmaceutical Co. located in Qazvin, Iran.
Conflicts of Interest
The authors declared that they have no competing interests.
Received: 2023/04/24 | Accepted: 2023/07/23 | ePublished: 2023/09/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |