BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2037-en.html
2- Department of Microbiology and Virology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran ,
3- Immunology Research Center, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Tuberculosis (TB) is one of the top 10 causes of death worldwide, and millions of people are affected by contracting tuberculosis every year (1). The World Health Organization (WHO) report (2021) showed that approximately 10 million people per year were infected with Mycobacterium tuberculosis (Mtb) worldwide between 2018 and 2020, and 1.4 million died in 2019 (2). TB infection outcomes reflect the innate ability of the human immune response to restrict M. tuberculosis infection and the insufficiency of immunological response (3). Fortunately, a few individuals infected with M. tuberculosis will develop the overt disease, and ∼90%–95% of infected individuals will stay healthy throughout their lives (4). About 5 to 10% of infected people, especially those with a weak immune system, will develop TB infection without treatment (Figure 1) (5). The WHO (World Health Organization) defines Latent tuberculosis infection (LTBI) as a persistent immune response to M. tuberculosis antigens without active clinical evidence. For a long time, the mainstream LTBI hypothesis was a tight balance between the host immune response and pathogen metabolism; and the active illness develops when this balance is broken. Furthermore, 1.7 billion people worldwide have a latent tuberculosis infection, and they are at risk of developing active TB disease (aTB) during their lifetime (6). People with LTBI are indeed a key source of new aTB cases (Figure 2). For this reason, if people with LTBI are not diagnosed and controlled, a significant percentage of people will develop aTB. There is no valid standard test for identifying LTBI. Current tuberculosis diagnostic methods are based on detecting an immune response to mycobacterial antigens injected into the skin or in vitro simulations of interferon-gamma release assays (IGRA) (7). Both tests have low sensitivity, which cannot distinguish between latent tuberculosis infection and active tuberculosis disease. Table 1 lists the characteristics of skin tests used to diagnose latent tuberculosis infection. Therefore, various techniques, such as alternate cytokine detection and employing different antigens, are being studied to increase the accuracy of these tests (8). In recent years, new M. tuberculosis stage-specific antigens have been used to diagnose latent and active tuberculosis infection. Using novel antigens for detecting latent tuberculosis infection and progression of tuberculosis disease can identify individuals to target preventive therapy and potentially predict therapy efficacy (9, 10). Novel M. tuberculosis antigens have been tested in previous studies. The majority of evaluated antigens, including Rv2029c, Rv2031c, Rv1733c, Rv2628, Rv0081, Rv2032, and Rv1737c are latency-associated antigens. These genes are found in the dormancy of the survival regulon (DosR), a genomic area containing around 50 genes expressed during latency (11, 12). Early detection and treatment of LTBI patients help reduce the burden of aTB, especially when the population screening is at high risk of developing active diseases. Risky people are those with human immunodeficiency virus (HIV) or other immune deficiencies, children under five, and household contacts with widespread exposure (of all ages) to someone with bacteriologically confirmed pulmonary TB (13). This review aims to assess current available diagnostic tools and evidence on novel M. tuberculosis antigens for LTBI diagnosis. It also selects the most promising antigens for future research.
Table 1. Characteristics of skin tests of diagnosis latent tuberculosis infection (LTBI)
| Conventional TST | Diaskin test | C-Tb skin test | EC-Test | |
| Antigens | PPD | ESAT-6/CFP10 | ESAT-6/CFP10 | ESAT-6/CFP10 |
| Positive internal control | No | No | No | No |
| Need for return visit | Yes | Yes | Yes | Yes |
| Time required for results | 48–72 h | 48–72 h | 48–72 h | 48–72 h |
| False positives with BCG vaccination | Yes | No | No | No |
| Cross-reactivity with NTMs | High | Low | Low | Low |
| Specificity | 95% | 98% | 99.3% | 98% |
| Sensitivity | 75% | 86% | 73.9% | 86% |
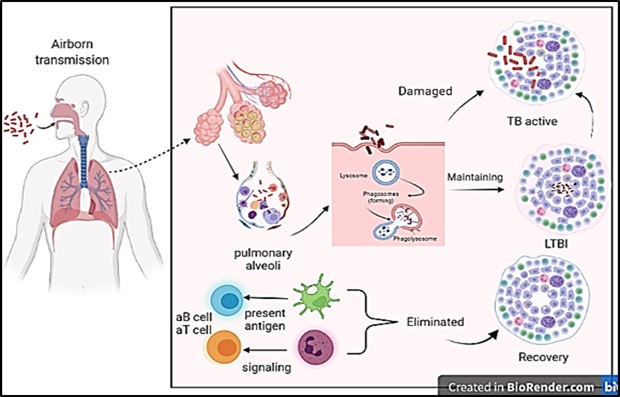
Figure 1. Diagram showing latent tuberculosis infection and its mechanism. Healthy people inhale M. tuberculosis (Mtb) excreted from active TB patients, which is recognized and phagocytized by macrophages, neutrophils, natural killer cells, and B lymphocytes. T lymphocytes form granulomas at the site of Mtb invasion after neutrophils release cytokines. A host with a strong immune system will clear M. tuberculosis and recover from the infection; In the event of weak immunity, M. tuberculosis will reproduce in the granulomatous tissue and break through the granulomatous restriction to cause active TB; If the host's immunity and M. tuberculosis' invasiveness are balanced, the host will be latently infected. Courtesy of the current publication.
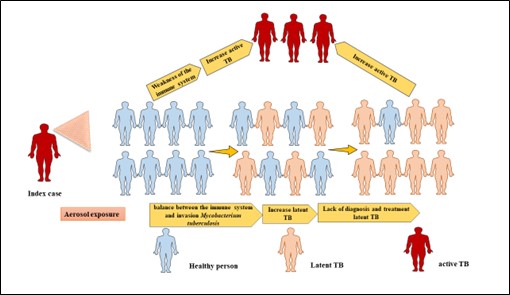
Figure 2. Progress spectrum of M. tuberculosis infection in individuals. Inhaling contaminated particles from a sick person can cause contamination in a healthy person. Due to the balance between the immune system and the invasion of Mycobacterium tuberculosis, some people enter the latent stage of the disease. People with latent tuberculosis are actually a key source of new cases of active tuberculosis (aTB). For this reason, if people with latent TB (LTBI) are not diagnosed and controlled, a significant percentage of people will develop aTB. Healthy people (blue), whereas individuals who test positive in the purified protein derivative (PPD) skin reactivity test and/or the IFN-γ release assay (IGRA) can either be asymptomatic (latent tuberculosis; pink) or be symptomatic with tuberculosis (aTB; red). Courtesy of the current publication.
Latent Tuberculosis Infection Diagnostic Methods
Clinical symptoms of LTBI are determined primarily by the host's positive immune system to M. tuberculosis antigens. People with LTBI have negative bacteriological results based on WHO guidlines. For this reason, the diagnosis is based on a positive tuberculin skin test (TST) or blood test, such as an IGRA. Table 2 shows different types of IGRA-based methods. However, screening procedures for LTBI vary according to national standards. The European Respiratory Society recommends IGRA or TST without bacillus Calmette-guérin (BCG) vaccination history. Also, medical history, physical examination, chest X-ray, and TST are used in France to detect LTBI infection (Figure 3).
Table 2. Characteristics of diagnostic methods for Latent tuberculosis infection based on whole blood interferon-gamma assay
| T-SPOT.TB | QFT-GIT | QFT-Plus | LIAISON QFT-Plus | LIOFeron TB/LTBI | |
| Antigens | ESAT-6 ,CFP10 | ESAT-6, CFP-10, TB7.7 |
ESAT-6, CFP-10 Shorter peptide |
ESAT-6, CFP-10 | ESAT-6, CFP-10, Ala-DH |
| Tubes | One tube | Nil, Antigen, Mitogen |
Nil, TB1, TB2, Mitogen |
Nil, TB1, TB2, Mitogen |
PC, TB-A, TB-B, NC |
| Technological platform | ELISPOT | ELISA | ELISA | CLIA | ELISA |
| Sample | PBMCs | Whole blood | Whole blood | Whole blood | Whole blood |
| Positive internal control | PHA | Mitogen | Mitogen | Mitogen | Mitogen |
| Outcome measure | IFN-γ producing T cells | IFN-γ produced by CD4+ T cells |
IFN-γ produced by CD4+ and CD8+T cells |
IFN-γ produced by CD4+ and CD8+T cells |
IFN-γ produced by CD4+ and CD8+T cells |
| Sensitivity | 83.5% | 89% | 91% | Unknown | 90% in aTB ,94% LTBI |
| Specificity | 76.2% | 99% | 95% | Unknown | 98% in aTB, 97% LTBI |
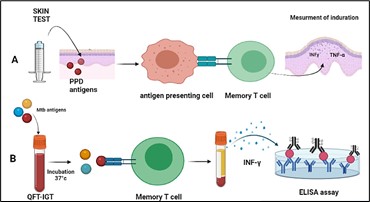
Figure 3. In vivo and In-vitro tests of detection Mycobacterium tuberculosis. A: According to the CDC, the Mantoux test involves injecting 5 tuberculin units (TUs - 0.1 ml) intradermally on the left forearm flexor surface. A tuberculin syringe should be used for the injection. The test result is read after 48. Individuals who have been exposed to bacteria in the past develop an immune response to bacterial proteins on their skin. The response is a classic example of a delayed-type hypersensitivity reaction (DTH). Inflammation is generated by T cells and myeloid cells attracted to the site of reaction within 1–3 days. B: Interferon Gamma Release Assay (IGRA) tests; Each patient receives a positive control tube, a negative control tube, and TB antigen tubes. A human blood sample (heparinized) is obtained from the patient by venipuncture, and one mL from each sample is pipetted into three tubes: negative, positive, and TB antigen. The tubes are gently mixed by shaking them upside down and placed in a 37°C incubator overnight. Next, the plasma is carefully removed and analyzed using human IFN-γ ELISA, which quantitates the amount of IFN-γ produced in response to the antigens from M. tuberculosis. Courtesy of the current publication.
Tuberculosis antigens as diagnostic tools
LTBI diagnosis depends on the cellular immune response to M. tuberculosis antigens. As the first-responding mediators against M. tuberculosis infection, macrophages absorb and destroy infected mycobacteria while activating adaptive immune responses capable of producing cytokines or chemokines and presenting antigens. Mucosal immunity also plays an essential role in infection suppression (7). Upon M. tuberculosis infection, T cells mediate the early-stage cellular immune response, whereas B lymphocytes mediate the humoral immune response in the middle and late stages of the illness. In the early stages of tuberculosis infection, IgM antibodies dominate, while subclass IgG antibodies predominate in the middle and late stages. IgG antibodies are produced in significant quantities in patients with latent or long-term illnesses. Most current diagnostic techniques focus on IgG and IgM antibodies produced by Mycobacterium tuberculosis-specific proteins (14). Several investigations have been conducted to determine whether M. tuberculosis secretory proteins are immuno-dominant and early indicators of TB illness (15). These studies have focused on the coding sections of the region of differences (RD), which is missing in M. Bovis, and different M. Bovis BCG gene fragments that play critical roles in the immunological protection given by this strain (16). LTBI is currently detected using the IGRA with antigens from Mycobacterium tuberculosis's RD-1 region (17). The EC-Test, C-Tb skin test, Diaskin test, QuantiFERON-TB Gold In-Tube (QFT-G-IT), T-SPOT TB, LIAISON QFT-Plus, and QFT-Plus were developed using RD1-encoded antigens such as 10-kDa culture filtrate protein (CFP-10) and 6-kDa early-secreted target antigen (ESAT-6). Researchers discovered 129 RD-associated antigens within M. tuberculosis' 16 RDs (18, 19). Furthermore, earlier research demonstrated that these antigens exhibit substantial changes between the BCG strain and M. tuberculosis despite excluding four antigens, including Rv2031c, Rv1737c, Rv1736c, and Rv2626c, from the RD areas. As a result, all RD-associated antigens should be considered while developing novel approaches for LTBI differential diagnosis. Previous research has suggested that IGRAs can be developed further by using various antigens derived from M. tuberculosis RD-associated and latency-associated antigens (19). The following is a description of the RD and latency areas, as well as the linked antigens. Table 3 lists the properties of classical and non-classical antigens of Mycobacterium tuberculosis H37Rv.
Table 3. Immunodominant antigens and Latency- associated antigens Mycobacterium tuberculosis H37RV
| Description | Gene name |
| Immunodominant antigens | |
| Secreted antigen 85-A (FBPA/85-B (FBPB) | Rv3804c/RV1886c |
| Heat shock protein 65 (HSP65) | Rv0440 |
| 6-kDa early secretory antigenic target (ESXA; ESAT-6)/10-kDa culture filtrate | Rv3875/Rv3874 |
| Low-molecular protein antigen (ESXH - TB10.4) | Rv0288 |
| Latency-associated antigens | |
| Redox balance, metabolism and energy | Rv0573c, Rv2007c, Rv1812c, Rv1734c, Rv3130c, Rv2003c, Rv2006, Rv1998c, Rv2029c |
| Nitrogen metabolism | Rv1736c, Rv1737c, Rv2032, Rv3127, Rv3131 |
| Nucleotide metabolism and repair | Rv2631, Rv2630, Rv0571c, Rv0570 |
| Protein synthesis and cell wall synthesis | Rv0079, Rv0574c |
| Sensor kinases and transcription regulators | Rv3132c, Rv3133c, Rv0081 |
| Host–pathogen interaction | Rv2626c, Rv2004c |
| Membrane proteins (proteases and transport proteins) | Rv2625c, Rv1997, Rv1735c, Rv1733c |
| Stress proteins | Rv3134c, Rv2624c, Rv2623, Rv2028c, Rv2005c, Rv1996, Rv2031c |
| Probable transcriptional regulatory protein | Rv0081 |
| Conserved hypothetical protein | Rv1738 |
| Hypothetical protein | Rv3126c |
| Conserved hypothetical protein | Rv3128c |
Genomics of the Difference Region
LTBI-RD antigens derived from M. tuberculosis are a potential resource of specific antigens for immunodiagnosis and vaccine development. Comparative genomic analyses of the genomes of virulent M. tuberculosis H37Rv, attenuated M. tuberculosis H37Ra, virulent M. Bovis, and attenuated BCG vaccine strains of M. Bovis revealed several so-called RD among these organisms (20). The RD secretion system contributes to tuberculosis pathogenesis in various ways, including virulence, phagosomal maturation inhibition, and bacteria transmigration from the phagosome to the cytosol. This system inhibits the vital secretion of cytokines for immune cell activation, downregulating the functions of dendritic cells, macrophages, and T cells (21). After genomic studies, 129 coding sequences known as RD proteins were discovered in pathogenic M. tuberculosis H37Rv. Mahairas et al. Identify three RDs between Mtb and BCG; namely, RD1, RD2, and RD3. A series of genes that encode 11 proteins from RD1, 13 proteins from RD2, and 12 proteins from RD3 were predicted in these regions (22). None of the BCG strains had RD1 and RD3, while RD2 was absent in some. However, RD3 was not found in most isolates of M. tuberculosis (23). Experiments using peptide pools of proteins produced by all M. tuberculosis-specific RDs for immunological reactivity revealed that RD9, RD7, and RD1 included immunodominant antigens recognized by T cells from TB patients (24).
Genomics of the Region of Difference-1 (RD-1)
RD-1 is a 9.5 kilobase M. tuberculosis genomic fragment (Figure 4) (25). Cole et al. The RD-1 region of M. tuberculosis was identified as nine genes ranging from Rv3871 to Rv3879c. The evaluation of ORF genes for mRNA and protein expression revealed at least of expression in M. tuberculosis. In addition to ESAT-6 (EsxA) and CFP-10 (EsxB), major T cell antigens have been identified as PE35, PPE68, Rv3871, Rv3879c, and Rv3878 (26). ESAT-6 (EsxA), CFP10 (EsxB), Rv3873 (PPE68), Rv3872 (PE35), Rv3871, and Rv3879c are among the RD1 proteins proposed to be used in detecting tuberculosis utilizing T cell tests (27). Furthermore, RD1 antigens have been tried in tuberculin-type responses with promising results. Apart from the protective function of the entire RD1 chromosomal region, animal studies of specific RD1 proteins have revealed the promise of ESAT6, CFP10, PE35, and PPE68 in developing novel subunit vaccines for tuberculosis (28).
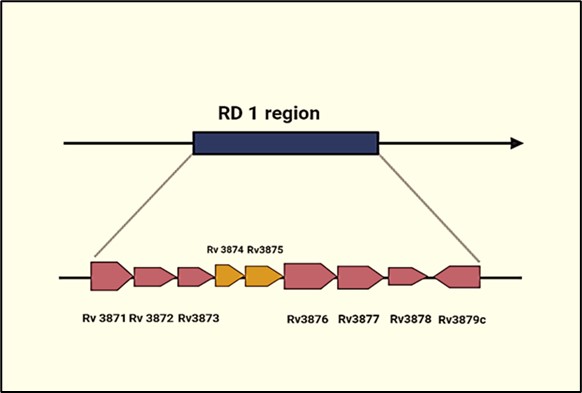
Figure 4. RD-1 region of Mycobacterium tuberculosis. Mycobacterium tuberculosis virulent strains have a 9.5-kb deletion region called the region of deletion (RD1), but all attenuated Mycobacterium bovis BCG vaccine strains do not have the deletion region. This region codes for at least nine genes. At least two RD1 gene products, ESAT-6 (Rv3875) and CFP-10 (Rv 3874), represent potent T- and B-cell antigens. Courtesy of the current publication.
Rv3874 and Rv3875 proteins
M. tuberculosis has a complex and lipid-rich cell wall with five expressed VII secretion systems optimized to effectively export material from the bacterial cytoplasm to the extracellular space (29). Rv3874 and Rv3875 encode two low molecular weight secretory proteins, CFP-10, and ESAT-6 (29). RD-1 promotes CFP-10 and ESAT-6. ESX-1 transfers a heterodimeric protein complex containing ESAT-6 and CFP-10 to the surrounding milieu (30). The heterodimer produced by ESAT-6 and CFP10 may require chaperone action mediated by the ATP-dependent protein Rv3868. This is because the expression of this protein is required for tight linkage and secretion (31).
Sørensen, Nagai (32) isolated ESAT-6 from the cytosol of mycobacteria as a protein with a molecular weight of ESAT-6 is released early during M. tuberculosis or M. Bovis infection (32). ESAT-6 protein of M. tuberculosis is a highly immunogenic protein in tuberculosis diagnostics and subunit vaccine production. ESAT-6 immunological investigations, biochemically isolated M. tuberculosis from short-term culture filtrates, revealed a significant M. tuberculosis antigen recognized by T cells among Mtb-infected mice (33). Studies with overlapping synthetic peptides showed that ESAT-6 is a significant T-cell antigen (34). In addition, ESAT-6 with several T cell epitopes, is recognized by T cells in conjunction with several human leukocyte antigen (HLA) class II molecules, which are often expressed in individuals in various geographical regions. Major histocompatibility complex (MHC) class II molecules on antigen-presenting cells deliver protein antigens to CD4+ T lymphocytes. These findings were supported by recombinant antigens and synthetic peptides of ESAT-6 (35). Infected people develop Th1 cells producing interferon‐gamma (IFN-γ), lymphotoxin (LT), and tumor necrosis factor (TNF-γ), which resist infection, but Th2 cells producing interleukin IL-4, IL-5, and IL-13 enhance disease susceptibility (36). The ESAT6-like proteins expressed by genes in RD1, RD7, and RD9 have low molecular weight, comparable genomic structure, and similar molecular weight with a few sequence similarities, about 6 to 20% with ESAT6 and CFP10 (37). CFP-10 comprises a C-terminal sequence, which secretes the complex from the bacterial cytoplasm. The complex is thought to dissociate under acidic circumstances, such as a phagolysosome (38). The CFP-10 gene is found in the same operon as the ESAT-6 gene. The two genes are members of the ESAT-6 family of small proteins with 40% sequence similarity. ESAT-6 and CFP10 are secreted as heterodimers, and ESAT-6 disrupts planar membranes (39). Renshaw et al. demonstrated that CFP10 and ESAT6 might be active biological molecules in vivo when forming a 1:1 heterodimeric complex. CFP10 plays an essential role in M. tuberculosis virulence, and the CFP10/ESAT6 complex attenuates the innate immune response by inhibiting the production of IL-12 and TNF-α from macrophages (40). Guo et al. reported that CFP10 induces the release of TNF-α from J774 macrophages (41). In addition, CFP10 is identified by murine CD8+ T cells following infection in vivo, and the immunological response to CFP10 can be a key component of M. tuberculosis host immunity. The role of CD8+ T lymphocytes in tuberculosis is unclear, and studies of mice with targeted deletion of cytotoxic granule proteins have yielded contradictory results. CD8+ T cells have significant cytolytic activity against infected macrophages. This is difficult to demonstrate ex vivo, and initial stimulation of the T cells in vitro leads to lysis of target cells. Studies demonstrated that CFP10-specific CD8+ T cells were cytolytic in vivo. CFP-10 stimulation of neutrophils resulted in a transient release of Ca2+ from intracellular stores, accompanied by neutrophil chemotaxis and reactive oxygen species (ROS) production (42).
PE/PEE Region
Deciphering the M. tuberculosis genome biology revealed that roughly 10% of the M. tuberculosis genome coding capacity encodes proteins with PE or PPE motifs in the N-terminus region. PE/PPE proteins in RD regions have recently been suggested as promising TB vaccines and diagnostic options. They both have Pro-Glu (PE) or Pro-Pro-Glu (PPE) motifs at the N-terminus (43). PE/PPE proteins include multiple repeating sequences and immunogenic regions, making them a rich source of B- and T-cell epitopes. At least 20 PE/PPE proteins induce CD4+ and CD8+ responses whether administered as complete recombinant proteins or as individual peptides (44). Several genes are arranged within the genome in a specified operonic pattern with a PE gene followed by a PPE gene. A few genes are related to members of the ESX family, which are major virulence factors for T-cell antigens (45). PE-PPE/PGRS family members are membrane-attached and localized to the cell surface, interacting with pathogens. Some family members have a role in regulating the immune system of the host. Two members of the PE–PPE family of proteins, namely PE35 (Rv3872) and PPE68 (Rv3873), are present at this locus (46). PE35 is secreted and necessary to express CFP-10 and ESAT-6, suggesting its role in their regulation (47). PPE68 is mostly cell wall-associated, interacting with the RD1 locus proteins Rv3866, Rv3868, CFP-10, and ESAT-6 (46). Mahmoudi et al. evaluated the IGRA. Stimulated with PE35 and PPE68. The results showed that IGRA had 76% and 80% sensitivity and specificity in children after stimulation with recombinant PE35. Moreover, 73% and 75% of this group responded to recombinant PPE68. M. tuberculosis proteins such as PPE17 (Rv1168c) generate significant B-cell and T-cell responses in inactive TB patients (48).
Rv0934 (38-kDa Antigen)
Rv0934 (the mycobacterial PstS1 antigen) belongs to the ABC transporter gene family as the phosphate-binding component of the M. tuberculosis inorganic phosphate absorption system (49). PstS1 is a glycosylated lipoprotein detected intracellularly, which is released into the culture supernatant. Furthermore, PstS1 has been identified as an immunodominant antigen with antibodies capable of distinguishing dormant TB from aTB (50). Xiao et al. assessed the diagnostic effectiveness of T-spot and ELISA assays using MPT64 and PstS1 in wild and mutant forms. The polymorphism of MPT64 and PstS1 proteins has almost no effect on the humoral immunity elicited by the proteins. As a result, there is no difference between wild-type and mutant proteins in ELISA tests for detecting patients. Therefore, MPT64 and PstS1 are plausible candidates for diagnostic antigens for the M. tuberculosis T-SPOT test at least when combined with other proteins. PstS1 showed higher immunoreactivity in LTBI than in aTB (51). PstS1 expands CD4+ and CD8+ memory T cells, amplifies secretion of IFN-γ and IL-22, and induces IL-17 production by effector memory cells in an Ag-unrelated manner in vitro and in vivo (52). Silva et al. followed up 120 patients for one year and measured interferon-gamma levels in their blood. They found that the composition of full PstS1 with CFP-10 induced IFN-γ response levels was similar to CFP-10- ESAT6 stimulation. They also suggested that this stimulation is higher in patients with LTBI than in those with pulmonary tuberculosis as a novel combination in IGRA (53).
Genomics of the Region of Difference (RD2, RD7, RD9)
RD2 Genes and Encoded Proteins
Antigens encoded in the M. tuberculosis differentiation region might be a source of uniqueness. RD2 antigens for immunodiagnosis, which encode 11 ORFs preserved in all virulent M. tuberculosis strains, were removed from BCG substrains produced from the original BCG Pasteur strain in 1926-1931 (54). MPT64 and CFP21 are immunodominant antigens that have been exploited to develop novel protective vaccines and diagnostic reagents. MPT64 (Rv1980c) encoded by RD2 is known to be a significant antigen. Moreover, MPT64 significantly combines with other M. tuberculosis antigens. MPT64 in diagnostic tests is recommended for M. tuberculosis, but there are some ambiguities due to its presence in some strains of the BCG vaccine. Therefore, MPT64 is proposed to diagnose TB such as IGRA and T. SPOT (55). A study utilized antigens rEC (recombinant of ESAT-6 and CFP10 fusion protein) and rCM (recombinant of CFP21 and MPT64 fusion protein) in enzyme-linked immunosorbent assays (ELISA) to detect tuberculosis in healthy people and household contacts' serum (56). Rv1985c is a potential transcriptional regulatory protein that encodes RD2. Rv1985c's immunogenic characteristics are unknown, excluding information from a transcriptional investigation (57).
RD7 Genes and Encoded Proteins
The M. tuberculosis-specific genomic region RD7 has eight ORFs (58). The combination of peptides corresponding to all eight proteins demonstrated that RD7 comprises immunodominant antigens activating the immune response with Th1 in human clinical trials with TB patients (59). EsxO (Rv2346c) and EsxP (Rv2347c) are two proteins encoded by RD7 genes, which belong to the ESAT6 family. EsxO (Rv2346c) and EsxP (Rv2347c) induced significant IFN-γ responses in tuberculin-positive in vitro (60). Lewinsohn et al. demonstrated that Rv2347c and a synthetic peptide library consisting of 15 mer Immunizations with EsxO (Rv2346c) and EsxP (Rv2347c) in mice using various delivery systems are antigens capable of stimulating IFN-γ secreting CD8+ T cells. They found both antigens induced protective Th1 responses but not pathologic Th2, Th17, or T regulatory cell responses. However, none of these antigens have been used to diagnose or develop a vaccine for human tuberculosis (61).
RD9 Genes and Encoded Proteins
The 5516 bp length contains seven ORFs (Rv3617 to Rv3623). Rv3619c (EsxV) and Rv3620c (EsxW) are ESAT-6 and CFP-10 family members. Immunological analysis of RD9 proteins with synthetic peptides revealed the encoding of immunodominant proteins (62). In M. tuberculosis culture filtrates, Rv3619c and Rv3620c are secretory proteins containing 94 and 98 amino acids, respectively. In silico research found them in Mycobacterium leprae, Mycobacterium avium, and Mycobacterium marinum (63).
Rv3619c and Rv3620c are members of a subfamily including Rv1198 Rv1197, Rv1038c Rv1037c, Rv2346c Rv2347c, and Rv1792 Rv1793 within the ESAT-6 family (64). Members of this subfamily have more than 90% amino acid sequence similarity to Rv3620c and Rv3619c. Synthetic peptide investigations have revealed that the Rv3619c and Rv3620c subfamilies contain potent T-cell antigens (65). The structure of Rv3620c-Rv3619c was predicted by molecular modeling and docking studies comparable to ESAT6-CFP10. Immunization with Rv3620c and Rv3619c proteins, alone or combined with other M. tuberculosis proteins, results in antigen-specific cellular and humoral immune responses in mice (66).
Latency Antigens
Dormancy of the Survival Regulation (DoSR)
During LTBI, M. tuberculosis is confined within granulomas produced by activated macrophages and other host components to organize the infected cells and create an environment to inhibit M. tuberculosis reproduction (67). Bacilli should adapt to various environmental stressors such as low oxygen tension, iron restriction, nutritional deprivation, low pH, and the generation of host factors like nitric oxide and carbon monoxide (68). During an infection, M. tuberculosis antigens change according to the ongoing pressure exerted by the host immune response. This is accompanied by the expression of many latency-related genes. The first reaction of M. tuberculosis is encoded by the dormancy survival regulon (DosR, known as DevR), which leads to the induction of 50 genes, many of which have unclear functions. The DosR protein, a response regulator, regulates these genes (67).
Role of DoSR Regulation in Latency
The DosR regulon, which is controlled by two sensor-kinase systems, DosS (Rv3132c) and DosT (Rv2027c), as well as a response regulator transcription factor (DosR (Rv3133c)) (69), is implicated in bacteria's stress response. DosT responds to stress (high NO and CO levels) by autophosphorylating at the 54th aspartate residue, accompanied by autophosphorylation and activation of DosS (70). Therefore, the DosR transcription factor can be phosphorylated to bind to the DosR regulon recognition sequence and initiate transcription of latency-associated genes. DosS and DosT contain a histidine kinase domain, including an ATP binding domain at the C-terminus and two GAF-A and GAF-B domains at the N-terminus (cGMP, adenylyl cyclase, FhlA). The sensor is activated when respiratory competitor molecules like NO and CO, abundant in LTBI, bind to the heme irons (in ferric form) of DosT and DosS to displace oxygen, which expresses DosR genes. DosR activation transcribes genes critical for obtaining energy from other carbon sources, allowing the bacterium to adapt to its new environment (70, 71).
Analysis of DosR Genes' Functional Properties
Dormancy survival regulon encodes antigens as latency-related antigens due to their upregulation during the M. tuberculosis dormancy state. These latency-related antigens are highly immunogenic in Mtb-infected individuals as potential LTB biomarkers for improving current T-cell-based IGRAs for active and LTBI diagnoses (72). Once M. tuberculosis exits the hypoxic non-respiring condition, the DosR regulon is critical for a quick restart of growth via engaging resuscitation-promoting factors (Rpf). M. tuberculosis includes five Rpf-like proteins in resuscitating this microorganism from dormancy to reactivation via a process involving Rpfs and associated proteins hydrolyzing the peptidoglycan. The DOS regulon contains protein Rv2029c (pfkB), which is increased in macrophages and during hypoxia (71).
Researchers found that Rv2029c-treated macrophages activated T cells followed by an increase in interferon-gamma and interleukin 2 secretion due to repeated polymerization of CD4+ and CD8+ T cells (73). Furthermore, more recent research has revealed that several DosR proteins, such as Rv2628, are recognized by aTB patients and LTBI. Rv2628 demonstrated much greater specificity than Rv2029c in both aTB patients and uninfected healthy participants. These results suggested that Rv2628 might be applicable as a biomarker to distinguish TB from LTBI and uninfected individuals (74). RV2626c, another DosR regulon gene boosted TNF-α and IL-12 in RAW macrophages by activating the NF-κB downstream signaling pathway and robustifying the IFN-γ response in aTB patients. One of the most highly elevated genes was Rv2031c, involved in cell wall thickening and stability under hypoxia (75). Rv2031 is one of the proteins, vital for Mtb's long-term survival, produced during LTBI. Rv2031c has a solid ability to stimulate T cells to produce an immune response with a certain antigenicity (76). Coppola et al. discovered that most regulation of in-vivo expressed M. tuberculosis (IVE-TB) genes occurs during in-vivo lung infection. Thirteen out of these 27 IVE-TB antigens induced increased levels of IFN-γ and IP-10, as well as TNF-α, IL-17, and IL-13. Therefore, Rv2029c, Rv2031c, Rv2034, Rv2628, and Rv3353c have been described as Mycobacterium tuberculosis latency-associated antigens (LAA). All LAA induced a greater mean IFN-γ response except for Rv3353c. As a result, "latency antigens" are novel Mtb antigens, which could be used in LTBI diagnosing tests (64).
Other Mycobacterium tuberculosis antigens
Studies have reported several proteins and biomarkers secreted by MTB as suitable candidates for diagnostic tests. MPT64 (Rv1980c) is encoded by RD2, known as the dominant antigen. Moreover, MPT64 significantly affects the combination with other Mtb antigens. Applying MPT to diagnostic tests for Mtb is recommended, but there are some ambiguities due to some BCG vaccine strains. Therefore, MPT is proposed to be used in TB diagnosis using T cell assays, such as IGRA and T. SPOT (77). The surface-secreted antigen such as Heparin-binding haemagglutinin (HBHA, Rv0475) is involved in extrapulmonary dissemination, inducing strong IFN-γ production by circulating CD4+ and CD8+ T lymphocytes in healthy individuals with LTBI but not in those with aTB (78).
This antigen can discriminate between these two groups of individuals infected with M. tuberculosis. However, HIV infection decreases IFN-γ expression in CD4+ T-cells in response to HBHA (79). In murine models, immunization with HBHA induces IFN-γ-producing, cytotoxic, and microbicide T lymphocytes and protects them at levels equivalent to those induced by BCG. The HBHA-specific IFN- γ production by the CD8+ T lymphocytes and NK cells depends on the presence of CD4+ T lymphocytes (80). Sammy Place et al. showed that CD41CD25 regulatory T (Treg) cells specific for HBHA are present at high levels in peripheral blood mononuclear cells (PBMC) from TB patients and that their depletion increases the HBHA-specific IFN- γ response to levels equivalent to those in PBMC from LTBI subjects. nHBHA is a methylated protein, whereas rHBHA produced in Escherichia coli (E. coli) is not methylated. Previous studies have reported that the IFN-γ response to nHBHA is significantly higher than that to rHBHA in latently infected healthy subjects. Consequently, HBHA is now considered a potent diagnostic antigen for detecting LTBI, which can be added to commercial IGRAs to enhance differential diagnostic performance (81). According to Villar Hernández, the presence of EspC (Rv3615c), EspF (Rv3865), and Rv2348-B in the QFN-G-IT improved the test sensitivity (82). Rv2346/47c- and Rv3614/15c-induced interferon-gamma in additional Rv3875 and Rv0129c significantly enhanced IFN-γ+ TNF-α+ with double-functional CD8+ T cells, which can be used as new antigens in serological tests such as interferon-gamma release assay and T.spot (83, 84). Mehaffy detected M. tuberculosis peptides in serum extracellular vesicles from TB patients using multiple reaction monitoring mass spectrometry (MRM-MS) assays. In this method, 40 M. tuberculosis peptides were identified from 19 proteins, mainly produced in the serum vesicles of patients with tuberculosis. This research finding showed that peptides RIP and DVL from GroES were identified in 17 active tuberculosis and five latent tuberculosis-infected people. Therefore, peptides can be used to diagnose latent tuberculosis in serological tests (85). Protein Rv2204c has a role in the pathogenesis of TB by interacting with host cell macrophages. This antigen specifically stimulates CD8+ cells and increases secreted interferon-gamma due to increased cell stimulation. It was measured in 72% of latent tuberculosis patients and 24% of pulmonary tuberculosis patients. This analysis showed that TNF alpha levels were higher in a patient with pulmonary tuberculosis than in one with latent tuberculosis (86). Rv3615c is recommended as an immune cell stimulator for tuberculosis due to the similarity of sequence and CFP-10 and ESAT-6 antigen size. This protein is observed in active and LTBI (87). According to Doddam et al., Rv2004c generates robust humoral and cell-mediated immune responses. Rv2004c has immunogenic capacity in latent tuberculosis-infected Japanese people. TNF-α, IL-8, IL-1, IFN-γ, and IL-12 were released dose-dependent by human peripheral blood mononuclear cells treated with Rv2004c at concentrations ranging from 10 ng to 1 g Rv2004c due to stimulate a significant IFN-γ response in LTBI patients (75). Rv1768 from RD14 was significantly conserved as a secreted protein among M. tuberculosis H37Rv. According to studies by Yuan et al., Rv1768 causes a significant level of iNOS and pro-inflammatory cytokines. In H37Rv-infected mice, Rv1768 stimulated the production of IFN-γ by peripheral blood mononuclear cells and bone marrow-derived macrophages, but not by BCG-infected or normal animals. Rv1768 can be used as a new TB diagnostic agent using ELISPOT and QFT-GIT (87).
Zhongchen Ma et al. Assessed 17 tuberculosis-specific proteins and reported that Rv1987, Rv3807, PPE57, Rv0220, and MPT64 proteins alone and combinations of Rv1987 + Rv3807, 16kDa + Rv0220, and MPT64 + Rv1986 tested in IGRAs displayed a significant correlation with the positive control constituted by a cocktail of ESAT-6 + CFP-10 + TB7.7. Rv0220 alone or the combination of Rv1987 + Rv3807 as the most potent stimulators in IGRAs. These proteins can be tested as alternative antigens in interferon-gamma release assays and T. SPOT tests (15). In LTBI, protein-containing fractions of Peptidyl-prolyl cis-trans isomerase A (PpiA) generated a considerably stronger interferon-gamma response than in inpatient tuberculosis. In LTBI, PpiA-specific IFN-γ/TNF-α exhibited 86% positive, compared with 18% inpatient tuberculosis. Unlike QFT-GIT, the PpiA-specific immune response was mainly limited to LTBI (88, 89). Rv0674 is a novel M. tuberculosis antigen. This protein performed well in measuring immunoglobulin G (IgG) while showing poor sensitivity and specificity for IFN-γ tests. Yang suggested that Rv0674 may be an appropriate candidate for developing TB serological diagnosis (90).
M. tuberculosis is a major cause of death worldwide as a primary global health concern. It is responsible for millions of deaths each year and it is estimated that by 2030 the number of deaths due to M. tuberculosis will increase to 10 million. Most people infected with M. tuberculosis do not develop TB, which can be attributed to an appropriate immune response to the infection. These responses can provide new insight into the defense mechanisms of the immune system against risk factors associated with aTB progression. Cellular immune responses mediated by IFN-γ-producing CD4+ T lymphocytes have long been established as an essential component of protective immunity against M. tuberculosis in both animal models and humans. Currently, M. tuberculosis proteins including CFP-10, ESAT-6, and TB7.7 are used as antigen stimuli in commercially available IGRAs to detect M. tuberculosis infection. While IGRA specificity for M. tuberculosis infection is often significant, IGRA sensitivity estimates vary widely and are often suboptimal. Based on data from published studies, including primary subjects with culture-proven M. tuberculosis infection, IGRA sensitivity estimates range from 70 to 90%. New antigens may improve analytical and clinical sensitivity by increasing the number of epitopes available to activate lymphocytes. In addition, they may increase serum IFN-γ. Improving IFN-γ and other cytokines could help early diagnosis of LTBI and TB infections, which in turn allows for better treatment and reduced disease spread.
We would like to thank everyone who has contributed to this project. Each of the members has provided me with extensive personal and professional guidance.
Conflicts of Interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
These authors contributed equally. All authors conceived and designed the study. All authors contributed to manuscript revisions. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Received: 2023/06/6 | Accepted: 2023/09/9 | ePublished: 2023/11/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








