BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2036-en.html


 , Mabrouka Bouacha2
, Mabrouka Bouacha2 

 , Sana Besnaci3
, Sana Besnaci3 

 , Narimen Bensaci1
, Narimen Bensaci1 

 , Akila Abdi1
, Akila Abdi1 

 , Paul Schweitzer4
, Paul Schweitzer4 


2- Department of Biochemistry, Laboratory of Biochemistry and Environmental Toxicology, Faculty of Sciences, University Badji Mokhtar, Annaba, Algeria, Algeria ,
3- Department of Biology, Laboratory of Cellular Toxicology, Faculty of Sciences, University of Badji Mokhtar, Annaba, Algeria
4- Centre d'Etudes Techniques Apicoles de Moselle (CETAM) Lorraine, Laboratory of Analysis and Beekeeping Ecology, Guénange, France
In recent years, there has been a significant increase in bacterial resistance to several drugs. The prevalence and spread of multidrug-resistant bacteria threaten human health (1) Patients often receive antibiotic treatment failure for prophylactic or therapeutic purposes. Based on the above, more effective alternatives should be found to preserve human health against multidrug-resistant pathogenic bacteria. Natural products, known for their low cytotoxicity and powerful antibacterial effects, such as herbs, plant extracts, essential oils, and honey, represent the best solution to the issue of multidrug resistance. Indeed, honey is an excellent alternative to anti-infective chemotherapy (2). In addition to its nutritional benefits, honey has multiple biological effects such as antimutagenic (3, 4), anti-inflammatory, antibacterial, and antioxidant activity, etc (5, 6). Honey possesses a powerful antibacterial agent due to its high osmolarity, acidity, and especially hydrogen peroxide content (7). In honey, there are two mechanisms of antibacterial activity. The first activity comes from the hydrogen peroxide compounds, known as the peroxide-dependant pathway (peroxide antibacterial activity), which represents the main contributor to antibacterial activity. During the ripening process of honey, glucose oxidation, which emerges from the glucose oxidase (GOX), produces hydrogen peroxide (H2O2), which is the most important contributor to the antibacterial activity of honey (8, 9). Some conditions, such as temperature and sugar concentration, should be maintained at certain levels to preserve the hydrogen peroxide concentration in honey sufficiently high to protect certain pathogenic microorganisms by disrupting their metabolism through a biochemical pathway. Even when honey is diluted in water, it is still a potent topical wound-healing agent (10). On the other hand, another mechanism involving non-peroxide factors caused by lysozymes, phenolic acids, and flavonoids is related to the origin of plants and honeybees (11, 12). These factors resist light and heat, enabling honey to remain intact even after prolonged storage (9).
Honey also contains organic acids (gluconic acid) and inorganic ions (phosphate, chloride) responsible for its acidity. The high acidity of honey contributes to its antibacterial properties (11, 13). The pH of honey, ranging from 3.2 to 4.5 regardless of its origin, is crucial in inhibiting bacterial growth. This low pH also creates a hyperosmotic medium, which inhibits the growth of many pathogenic bacteria by absorbing their vital water and preventing their multiplication (8, 14).
It has been proven that honey exhibits a strong antibacterial effect on bacterial infections caused by multidrug-resistant bacteria (15-17). The antibacterial activity of honey has been tested against many pathogenic bacteria in different environments to treat wounds and burn infections. Also, it was tested against urinary tract infections in pregnant women caused by multidrug-resistant strains such as Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, etc. (8, 12, 18). Many factors control the chemical composition of honey, including botanical source, geographical location, season, collection area, environment, processing, and storage conditions (19). Honeybees produce honey from several floral sources corresponding to pollen taxa. The pollen of polliniferous, nectariferous, and anemophilous plants is often found in honey and reflects environmental factors and plant resources foraged by honeybees (1, 7). Despite the major importance of the pollen content in the geographical and botanical characterization of honey, the European :union: has not issued any specific legislation for it. However, the identification and evaluation of honey's botanical and phytogeographical sources are based on pollen and sedimentary constituents analysis. This analysis allows the certification of the obtained results (20, 21).
In recent years, there has been an increasing interest in determining the antibacterial effect of honey from different regions. Yet, the data available on the pollen profile and the antibacterial activity of Algerian honey are very few compared to Algeria's significant botanical, climatic, and geographical diversity. Its surface is geographically located between two floral empires (Holarctis and Paleotropis): including 3139 plant species, many of which honeybees use in honey production (22). Therefore, this study the qualitative melissopalynological analysis, GOX activity, and the antibacterial effect of five honey samples from various botanical and geographical origins.
Honey Samples
Across different areas (Table 1) and various botanical sources in Algeria during August 2019, five natural honey samples produced by the Apis mellifera honeybee were collected from beekeepers. The samples were preserved in airtight sterile glass containers and then stored in the refrigerator at 4°C until analysis. The antibacterial activity of honey samples was evaluated in six dilutions (80%, 40%, 20%, 10%, 5%, and 2.5%). The honey samples were filtered with sterile syringe filters (0.2 µm, Fisher Thermo Scientific).
Table 1. Harvested area and honey samples’ description
| Honey | Harvested area description | Honey description | ||||||
| City | Region | Area | Climate | Color | Odor | Flavor | pH | |
| H1 | Laghouat | The central part of the north of Algeria | Field | Arid | Light amber | Medium power, fruity | Fairly sweet, fruity, tangy | 3.78 |
| H2 | Annaba | Extreme Mediterranean north-eastern of Algeria |
Field | Humid | Dark amber | Quite powerful, fruity | Fairly sweet, very fruity | 4.20 |
| H3 | El Bayadh | West of Algeria | Field | Semi-arid | Dark amber | Average power, vegetable | Complex, fruity, tangy, menthol, quite persistent | 4.29 |
| H4 | Djelfa | The central part of the north of Algeria | Field | Semi-arid | Dark amber | Quite powerful, complex, menthol | Complex, fruity, quite acidulous, persistent | 4.63 |
| H5 | Algiers | Central Mediterranean part of north Algeria | Mountain | Humid | Dark Amber | Medium power, complex, "animal" | Complex, "animal", fruity, tangy | 4.89 |
Qualitative Melissopalynology Analysis
Qualitative melissopalynology analysis was carried out according to the method described by Louveaux et al. (1978). Briefly, 10 g of honey was dissolved in 20 mL of hot water (below 40°C), then centrifuged for 5 min. 10 mL of distilled water was added to the pellet resulting from centrifugation for 10 min at 3,000 rpm, transferred to a microscope slide, dried, and identified. Pollen grains were identified from the digital and bibliographic databases of the center's beekeeping analysis and ecology laboratory, France (CETAM). The preparations were examined at different magnifications (×100, ×400, and ×1000). Pollens from anemophilous plants or non-nectariferous plants were subtracted from the total number of pollens before calculating the percentages from nectariferous plants. The percentages of the obtained pollen are those from nectariferous plants only. If the dominant pollen rate of honey comes from a single flower species (greater than or equal to 45%), then the honey is monofloral (23, 24).
Bacterial Strains Isolation
The antibacterial activity of honey samples was tested against 78 multidrug-resistant bacterial strains. The pathogenic bacteria were isolated from infected wounds in the microbiology laboratory of the public hospital establishment, El Hadjar, Annaba, Algeria. The bacterial strains were isolated from infected wounds (burns, diabetic foot, and post-surgical wounds). The pathogenic bacteria were identified by conventional microbiology methods (Gram stain, oxidase test, and catalase test) and confirmed by the analytical profile index API 20E, API 20NE, and API STAPH (Bio-Mérieux, France). According to their antibiotic resistance profile, only bacterial strains showing multidrug resistance were selected. Tested antibiotics are those commonly used for the treatment of infections caused by E.coli, P. aeruginosa, and S. aureus, including amoxicillin-clavulanate, ceftazidime, ceftriaxone, ciprofloxacin, clindamycin, gentamicin, imipenem, oxacillin, tobramycin, trimethoprim-sulfamethoxazole.
Antibacterial Activity Assessment
Agar Well Diffusion Assay
Agar well diffusion assay was carried out according to the method described previously by Albaridi (2019). Mueller Hinton agar (Fisher Scientific, Bd Difco, Dehydrated Culture Media, USA) is inoculated using a swab soaked in bacterial suspension, adjusted to 0.5 Mc Farland turbidity (0.05 mL of barium chloride (1%) and 9.95 mL of sulfuric acid (1%). Wells of 6 mm diameter are perforated on the Mueller Hinton agar plate. Each well was filled with 50 µL of honey at a dilution of 50% (v/v). The plates were incubated at 37°C for 24 hours. During incubation, the honey diffuses into the agar creating a clear zone around the well, called the inhibition zone of bacterial growth. The diameter of the inhibition zone was expressed in millimeters; the inhibition zone size was measured to identify the antibacterial potency of the tested honey (25).
Broth Dilution Assay
The broth dilution method is used to determine the minimum inhibitory Concentration (MIC), which is defined as the lowest concentration of an antibacterial agent that inhibits the visible growth of a bacterium. In each test tube, 4.5 mL of the dilutions of honey were added to 4.5 mL of the bacterial suspension. The tubes were incubated at 37°C for 24 hours. The MIC corresponds to the absence of visual turbidity compared to the positive control (bacterial suspension).
To determine the minimum bactericidal concentration (MBC), 10 µL of each tube that has not shown any turbidity in MIC determination was inoculated on nutrient agar plates (Fisher Scientific, Bd Difco, Dehydrated Culture Media, USA) at 37°C for 24 hours. The MBC was the lowest concentration of honey that did not show bacterial growth on nutrient agar plates (25).
Glucose Oxidase Activity
The GOX activity was determined according to the method of Burgett (1974). This is based on the formation of color by an oxidized chromogen (o-dianisidine) in the presence of hydrogen peroxide (H2O2) and peroxidase. H2O2 was used as a standard (10-100 μmol/L) with peroxidase and o-dianisidine was used for the quantitative determination. A mixture of 0.7 mL of glucose (2.14 mM, dissolved in 100 mM sodium phosphate buffer, pH 6.1), 0.1 mL of ethanolic solution of o-dianisidine (1mg/mL), 1 mL of horseradish peroxidase, and 0.1 mL of honey solution (0.2 g/mL in a 100 mM sodium phosphate buffer, pH 6.1) was prepared. The mixture was incubated at 37°C for 30 minutes and then a volume of 0.1 mL of 1 M hydrochloric acid was added to the mixture. The crude protein extracts of honey were prepared by filtration with tap water (20°C) for 24 hours. The absorbance was measured at 400 nm and the enzymatic activity was expressed as μg H2O2/h g of honey (28,29).
Statistical Analysis
The data were analyzed using the SPSS version 26 software (IBM SPSS Statistics, Armonk, New York, USA). The data of the antibacterial activity (resistant, bacteriostatic, and bactericidal) of the different honey samples with different GOX activity levels against E. coli, P. aeruginosa, and S. aureus were expressed as percentages. They were analyzed statistically using the Chi-square test of association followed by a pairwise z-test post hoc analysis with Bonferroni Correction for multiple comparisons between groups (C 1936). Values of two-sided P≤0.05 were considered significant and highly significant when P<0.01. The effect size (v) measurement was concluded and the degree of freedom and interpreted as mentioned by Cohen (1988).
Quantitative Melissopalynology Analysis
The identification of honey samples and the results of the qualitative pollen analysis are listed in Table 2. Fifty pollen types corresponding to eighteen families have been identified. The most important found pollens are reported in Figure 1. The analysis of honey H1 shows that the dominant pollen is Hedysarum coronarium. Honey H2 does not contain dominant pollens, but the Rhamnaceae and the Apiaceae’s pollens were detected as secondary pollens. Honey samples H3 and H5 showed that the dominant pollen is the Apiaceae family and (Coriandrum type), respectively. A few isolated pollens existed in all honey samples. The different types of the family Brassicaceae pollens were presented in samples H1 and H3; they were minor pollens. A great variety of minor pollen characterizes the honey samples H2 and H3. The pollens of fruit trees (Prunus/Pyrus) exist in a very low percentage in all the honey samples except the honey H3 from the Saharan region. The anemophilous pollens or pollens of plants are considered non-nectariferous. They exist in all the honey samples except the H4. The most detected pollens are the pollens of Cistus sp then Olea Europaea. However, the pollen of the family Poaceae was detected only in honey H2.
Table 2. Characterization of pollen types in honey samples
| Honey samples | H1 | H2 | H3 | H4 | H5 |
| Dominant pollen (≥ 45%) |
Hedysarum coronarium | / | Apiaceae | Ziziphus spp | Apiaceae type Coriandrum |
| Secondary pollen (≥ 16% and < 45%) |
Brassica napus | Rhamnaceae Apiaceae |
Ziziphus spp | Brassica sp | / |
| Minor pollen (≥ 3% et < 16%) |
Lotus sp | Helianthus sp, Myrthaceae, Brassicaceae, ceratoniasiliqua, genista type | / | Erica arborea Carduus type lotus sp |
Brassica sp |
| Important minor pollen (< 3%) | Stachys type, Buxus sempervirens, Ranunculacea, Brassicacea, Prunus dulcis, Genista type, Ucalyptus sp, Medicago sativa, Fabaceae, Rosmarinus officinalis, Asphodelus sp, Asteraceae, myrthacea, Carduus type. |
Carduus type, Hedera helix, Convolvulacea, Chrozophora tinctoria, prunus/pyrus, Erica arborea, Asteraceae liguliflore, Trifolium sp, solidago type, |
Trifolium sp, Arctium type, Trigonella sp, Rubus sp, Brassica sp, Asteracea, Dipsacacea, Centaurea sp, Fabacea, | Xanthium sp, Asteraceae liguliflore, Apiaceae, Trifolium sp, prunus/pyrus, Arctium type | Erica arborea, Rubus type, Prunus/Pyrus |
| Very minority or isolated pollens | Cistus sp | Olea europaea, Cistus sp Poaceae | Olea europaea Cistus sp |
Cistus sp | / |
Figure 1. Light microscopy photographs of some pollen grains observed in honey samples (X1000). A: Ziziphus spp, b: Brassica napus, c: Lotus sp, d: Hedysarum coronarium, e: Carduus type, f: Cistus sp, g: Erica arborea, h: Apiaceae type Coriandrum
Antibacterial Activity
The results of the antibacterial activity of honey are shown in Table 3. The averages of inhibitory diameters were 13.88-15.90 mm, 15.25-18.67 mm, and 19.36-24.51 mm, for E. coli, P. aeruginosa, and S. aureus, respectively. There were no significant differences between the inhibitory diameters of the five honey; however, S.aureus strains seem to be more sensible than Gram-negative bacteria (E. coli and P. aeruginosa). The lower the MIC value, the more the honey sample has a strong antibacterial activity; hence, a very low concentration was sufficient to inhibit bacterial growth. The mean of MIC values were between 16.59 and 44.73 and the MBC values were between 16.61 and 83.84% (v/v). MBC/MIC ratios were between 0.73 and 3.75, which means that honey samples exhibit a bactericidal effect on the pathogenic bacteria.
Table 3. The Antibacterial activity of honey samples determined by broth dilution assay, MIC, MBC, and MBC/MIC ratios determination
| Pathogenic bacteria | Antibacterial effect | H1 | H2 | H3 | H4 | H5 |
| Inhibitory diameter (mm) | 14.25±0.56 | 15.73±0.54 | 13.88±0.48 | 14.31±0.52 | 15.90±0.50 | |
| MIC (% v/v) | 40.96± 0.05 | 40.67 ± 0.06 | 16.59 ± 0.06 | 41.71 ± 0.03 | 21.96 ± 0.39 | |
| E. coli (n=26) | MBC(% v/v) | 83.84 ± 0.05 | 54.71 ± 0.03 | 71.15 ± 0.03 | 64.53 ± 0.03 | 70.86 ± 0.04 |
| MBC/MIC ratio | 02.04 | 1.33 | 4.28 | 01.54 | 03.22 | |
| Inhibitory diameter (mm) | 15.25±1.51 | 15.92±0.98 | 16.25±1.50 | 17.73±1.23 | 18.67±1.41 | |
| MIC (% v/v) | 44.73 ± 0.39 | 18.84 ± 0.03 | 21.23 ± 0.04 | 19.01 ± 0.04 | 18.39 ± 0.03 | |
| P.aeruginosa (n=26) | MBC (% v/v) | 74.67 ± 0.04 | 31.61 ± 0.05 | 23.28 ± 0.05 | 37.95 ± 0.03 | 26.61 ± 0.04 |
| MBC/MIC ratio | 1.66 | 1.67 | 1.09 | 1.99 | 1.44 | |
| Inhibitory diameter (mm) | 21.34±1.53 | 19.36±1.34 | 20.63±1.71 | 21.69±1.64 | 24.51±1.70 | |
| MIC (% v/v) | 19.53 ± 0.05 | 26.41 ± 0.02 | 17.03 ± 0.05 | 17.75 ± 0.02 | 11.19 ± 0.04 | |
| S.aureus (n=26) | MBC (% v/v) | 65.31 ± 0.06 | 37.50 ± 0.05 | 25.78 ± 0.05 | 27.19 ± 0.03 | 25.47 ± 0.06 |
| MBC/MIC ratio | 3.34 | 1.41 | 1.51 | 1.53 | 2.27 |
Glucose Oxidase Activity
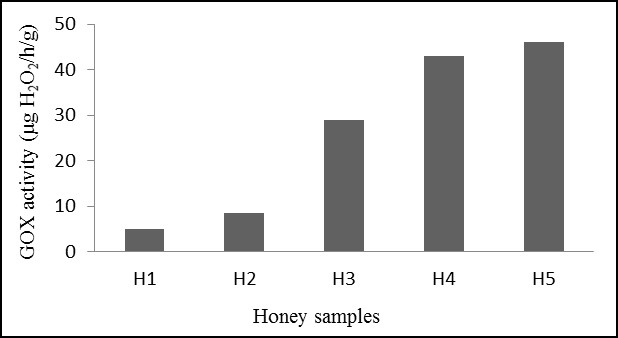
The results of the determination of GOX activity and its correlation with the antibacterial effect are reported in Figure 2 and Table 4 respectively. The result showed that honey samples from different floral sources have different GOX levels. This could influence directly the antibacterial effect of honey samples. In Table 4, the statistical analysis showed that there is a significant association between the bactericidal activity of honey on E. coli, P. aeruginosa, and S. aureus strains and GOX activity. However, only Honey samples H1 and H5 showed significant differences between bactericidal and bacteriostatic percentages in E. coli and S. aureus strains. There are also significant differences between the percentage of resistant bacteria and both the bactericidal and bacteriostatic percentages of H1 in P. aeruginosa strains.
Table 4. Correlation between the antibacterial activity of honey samples and its GOX activity
| Bacterial strain | Chi-Square Test | Honey sample | Percentage of bacterial strains (%) | ||||
| χ2 | v | P-value | Resistant | Bacterostatic | Bactericidal | ||
| E. coli (n=26) |
16.61* | 0.253* | 0.034 | H1 | 23.1 | 57.7 | 19.2 |
| H2 | 11.5 | 50.0 | 38.5 | ||||
| H3 | 19.2 | 38.5 | 42.3 | ||||
| H4 | 15.4 | 30.8 | 53.8 | ||||
| H5 | 15.4 | 15.4 | 69.2 | ||||
| P. aeruginosa (n=26) | 20.26** | 0.279** | 0.009 | H1 | 26.9 | 30.8 | 42.3 |
| H2 | 3.8 | 42.3 | 53.8 | ||||
| H3 | 0.0 | 42.3 | 57.7 | ||||
| H4 | 3.8 | 34.6 | 61.5 | ||||
| H5 | 3.8 | 23.1 | 73.1 | ||||
| S. aureus (n=26) |
18.795* | 0.269* | 0.016 | H1 | 7.7 | 61.5 | 30.8 |
| H2 | 0.0 | 38.5 | 61.5 | ||||
| H3 | 3.8 | 38.5 | 57.7 | ||||
| H4 | 0.0 | 26.9 | 73.1 | ||||
| H5 | 0.0 | 19.2 | 80.8 | ||||
The use of natural honey produced by Apis mellifera honeybees is considered an important part of traditional medicine. It has been used since ancient times for the treatment of many diseases, including burns and infectious diseases. The antibacterial property of honey varies considerably depending on its geographical, seasonal, and botanical source as well as harvesting, processing, and storage conditions (10, 19, 26).
As reported in Table 2, the melissopalynological method confirmed the identity of honey sources. This study defines the dominant botanical sources of Algerian honey produced in five regions with varied climates and botanical flora. Apis mellifera has used a wide spectrum of plants as pollen and nectar sources; 50 types of pollen from 18 families were identified in the studied honey samples. The high-represented families were Apiaceae, Fabaceae, and Rhamnaceae. The dominant pollen types in honey were Hedysarum coronarium, Ziziphus spp, and Apiaceae type Coriandrum. The honey's geographical origin and the collection area's environment can influence the properties of honey and its therapeutical effects (16). The physicochemical characteristics of plant compounds and bee-related factors varied in each honey type derived from the same botanical source. According to Louveaux et al. (1978), two honey samples can be classified as monofloral (H1: Hedysarum coronarium, H4: Ziziphus spp.), whereas honey H2, H3, and H5 are multifloral honey (24).
The evaluation of the antibacterial effect of honey has shown that all honey samples exhibit a good antibacterial effect against pathogenic bacteria. The inhibitory diameters in Table 3 varied significantly from 14.50±0.56 to 24.50±0.69 mm. These results are interesting compared to those reported by Agbagwa and Frank Peterside (2010), who tested the antibacterial activity of different honey samples from Nigeria. The mean of the inhibitory diameters was 4.4 to 17 mm for the same bacterial species (27). MIC values were between 16.59 and 44.73% (v/v) and MBC were between 16.61 and 83.84% (v/v). The MBC/MIC ratio was between 0.73 and 3.75. The determination of the MBC/MIC ratio is important to distinguish between honey that exhibits a bacteriostatic effect, which inhibits bacterial growth without killing the bacteria from honey that exhibits a bactericidal effect, which kills the bacterial cell. According to O'Neill and Chopra (2004), when the MBC/MIC ratio is less than or equal to 4, the antimicrobial agent has a bactericidal effect, therefore, all honey samples have a bactericidal effect on the pathogenic bacteria (28). Gram-positive bacteria were more susceptible than Gram-negative bacteria. This finding was reported by several authors (10, 12, 29, 30). Nevertheless, Al-Hasani (2018) reported that there were no significant differences in the efficiency of honey towards bacteria (31). Other authors have reported that Gram-negative bacteria were more sensitive to honey than Gram-positive bacteria (32-35). The differences in the antibacterial effect of honey could be related to the differences in the structure and composition of the membrane (36). The Gram-negative bacteria are surrounded by a thin peptidoglycan cell wall, which itself is surrounded by an outer membrane mainly composed of lipopolysaccharide (which consists of lipid A, core polysaccharide, and O antigen). This may affect the permeability of the membrane and reduce the diffusion of antibacterial agents into the bacterial cell. However, Gram-positive bacteria lack an outer membrane but are surrounded by layers of peptidoglycan many times thicker than that found in Gram-negative bacteria (36, 37). Moreover, the differences in the antibacterial effect of honey could be affected by the methods used to evaluate the antibacterial effect, as well as the level of susceptibility or resistance of the bacterial strains. Other factors related to honey samples such as bee species, geographical region, floral resources, and harvested and storage conditions could influence the antibacterial effect of honey (7, 19, 34, 38). On the other hand, the antibacterial effect of honey is due to various factors such as its acidity, high osmolarity, hydrogen peroxide (H2O2) content, and phytochemical components, which in a synergetic manner affect the growth and the viability of the pathogenic microorganisms (39, 40).
The results of the determination of the GOX activity in Figure 2 showed that it varied significantly from one honey to another. It seems to vary between honeys of different floral sources and different geographical origins. It is worth mentioning that the highest amount of GOX was found in the honey H5 from the Mediterranean region (Algiers). This may be due to the floral and geographical origin, which affect the pollen nutrition in the bee colony. Similarly, (41, 42) have suggested that floral resource diversity may have a direct effect on the antibacterial factors, including GOX activity. Table 4 shows that the GOX activity correlates with the antibacterial effect of honey samples. Indeed, the antibacterial effect of honey is mostly due to the presence of H2O2, which is produced by the GOX enzyme from the oxidization of glucose into gluconic acid and hydrogen peroxide. Therefore, the GOX enzyme is a critical enzyme in honey that indirectly contributes to its therapeutical properties (43).
This study demonstrated that honey samples from different botanical and geographical resources might have different levels of GOX activity, which has a direct impact on the antibacterial activity of honey. This could be related to the difference in the floral resources, which affects the nutrition of the bee. Therefore, the botanical origin of honey strongly influences its therapeutic properties. Further research on the composition of honey in bioactive substances and the mechanisms responsible for their biological activities could improve the treatment of infectious diseases particularly in developing countries.
None.
This research received no specific grant from any funding agency in the public or commercial sectors.
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2023/03/21 | Accepted: 2023/07/27 | ePublished: 2023/09/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |