BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2026-en.html
2- School of Medicine, Islamic Azad University, Tonekabon Branch, Tonekabon, Iran
3- Microbial Biotechnology Research Center, Iran University of Medical Sciences, Tehran, Iran ,
Climate change is widely recognized as the most significant global threat to public health of the 21st century, with potentially serious impacts on health predicted to show itself in fluctuating routes in various parts of the world, from the tropics to temperate zones (1, 2). Human and animal health can be influenced by climate variability in different ways: both directly, because of physical impacts that cause physiologic stress (e.g. temperature) or injury (e.g., floods, storms, fires, and droughts); or indirectly, because of social and ecological disruptions, like shifting patterns of disease vectors, crop failures, and human migration (3). The health effects of such impacts tend to reveal shifts in the seasonal and geographic patterns of human infectious diseases, and changes in the severity and frequency of outbreaks.
In the current century, the role of climate as a host- and pathogen-related risk factor for infectious diseases has gained attention (4). Some research has focused on how climate change can facilitate the spread of disease vectors to new areas, putting new populations at risk (4). It is known that weather conditions such as rain, floods, humidity, and heat waves can affect infectious diseases. For example, long periods of rain increase the possibility of the spread of vector-borne diseases (5). Longer seasons with mild temperatures may also increase the likelihood of vector-borne disease transmission. At warmer temperatures, vectors are more infectious and can transmit pathogens earlier in their lives. While rapid climate change has overshadowed human health by changing the epidemiology of highly pathogenic microorganisms, the models used in this field are still unable to provide accurate predictions (6). It has been predicted that by 2100, there will be an increase in average precipitation ranging from 1-9% and an average global temperature increase of 1.4-5.8°C (7). Some studies have shown a direct relationship between temperature and the number of infectious patients. In China, an increase in temperature has been documented in many districts, with an increase in warming from north to south (8). Investigations in the USA, Australia, Peru, and Europe have analyzed the link between climate variation and diarrheal diseases (7). Therefore, warm and unstable climate as well as prolonged global warming, create suitable conditions for the emergence, spread, and redistribution of infectious diseases in different geographical areas. This study presents a literature review on the scientific evidence for the effect of major climate variables and extreme weather events on human bacterial infectious diseases. To achieve this goal, relevant articles in PubMed and Scopus databases regarding the impact of global climate change on human bacterial infectious diseases were evaluated.
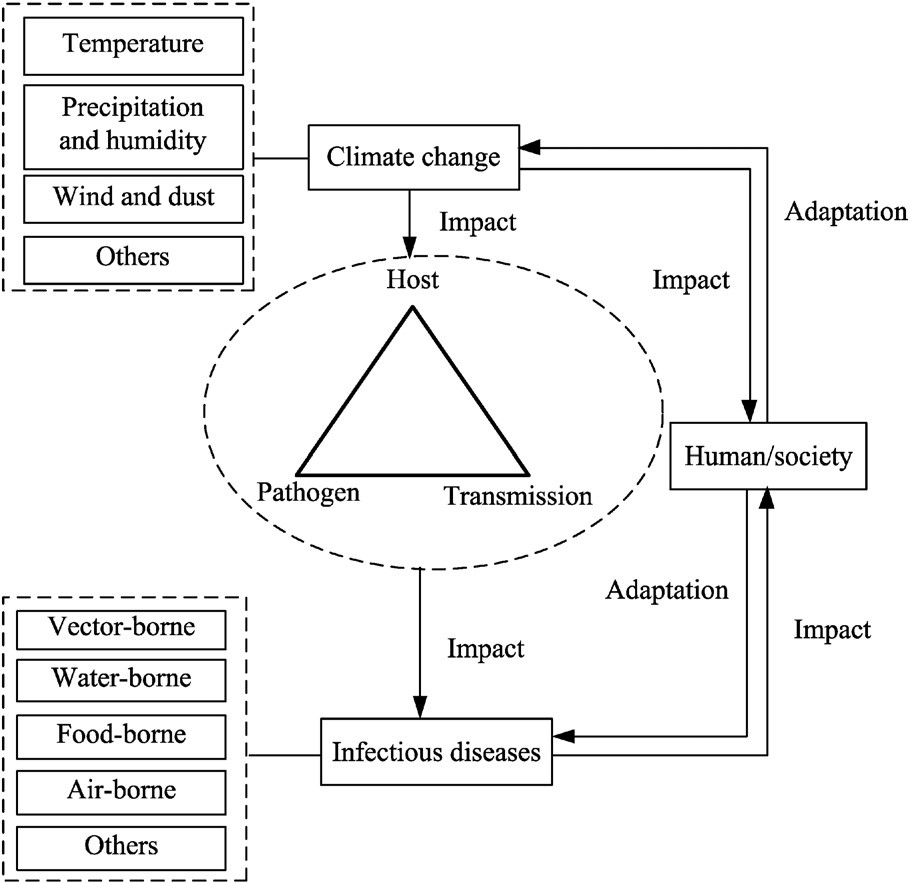
Climate change may influence the prevalence and geographic distribution of seasonal conditions, but often not in a predictable fashion. Temperature and relative humidity directly affect the survival of enteroviruses and the rate of replication of bacterial and protozoan pathogens (certain serotypes) in the environment. Moreover, these meteorological factors may likewise have a significant effect on the environmental reservoirs of infection as well as human behavior. Precipitation, especially heavy rainfall events, may influence the level and frequency of drinking water contamination. Every one of these components could result in an increased incidence of enteric infections. However, the effects of these meteorological factors are not predictable in published examinations, which could be because of population and ecological characteristics and different climatic conditions in various locales. Figure 1 shows the link between climate change, human infectious diseases, and human society.
Figure 1. Climate change, human infectious diseases, and human society.
According to data, there is a direct relationship between Gram-positive pathogens such as CoNS (Coagulase negative staphylococci (and temperature. This means that warmer temperatures are associated with increased density of Staphylococcus spp. and Enterococcus faecium (9). A study reported that the prevalence of Enterococcus cloacae in summer increased by 46% compared with winter (10). In addition, an increase in temperature was associated with a significant increase in the incidence of Staphylococcus aureus bloodstream infections (BSI) and a non-significant increase in the incidence of Enterococcus BSI, indicating the effect of temperature change on bacterial prevalence (11). On the other hand, influenza and pneumonia, the eighth leading cause of death in the USA, have higher incidences during the winter months (12, 13). Research has shown that more heat is associated with a lower density of Streptococcus pneumoniae, and the phenomenon of increasing pneumococcal infections in winter has been regularly reported. The prevalence rate of S. pneumoniae at 20°C is reported to be 35% lower than the prevalence rate at 5°C (9). Although the specific reason for pneumonia seasonality is indistinct, factors such as lower relative humidity, indoor crowding (resulting in closer contacts), indoor air pollution, association with other common seasonal respiratory pathogens (such as respiratory viruses), seasonal variation in the human immune system, and low ultraviolet radiation may contribute to the development of seasonal pneumonia (6). Furthermore, a study evaluating the prevalence of the disease versus environmental factors (humidity, mean temperature, and precipitation) in the Sylhet region of Bangladesh found a negative relationship between meningitis and pneumonia, and high humidity was inversely related to meningitis. Additionally, the high prevalence of meningitis was associated with low rainfall, and weather patterns had effects on disease incidence (14).
Climate changes including temperature and changing water patterns can influence the severity and incidence of respiratory infections, directly or indirectly, by affecting the host immune responses and the vectors,. Children and students seem to be at risk of temperature changes in the face of extreme weather fluctuations, severe storms, and drought. For example, a rapid drop in temperature has been shown to play a role in pediatric pneumonia in Australia (6, 15). Some studies show that there is a negative correlation between invasive pneumococcal disease and ambient temperature and others indicated that there has been an increased incidence of pneumonia during the spring and winter (16, 17). Despite the evidence for the role of rainy seasons in the development of pneumonia in Asia and Africa, as well as the reduction of the disease in the face of rising global temperatures, the relationship between environmental factors and pneumonia is complex and ambiguous. Temperature fluctuations appear to be very effective in the prevalence of pneumonia and mortality due to respiratory infections (especially in children and the elderly) (18). Regarding the study of temperature changes in the incidence of the disease, various indicators are used, including DTR (diurnal temperature range) and TCN (temperature change between two neighboring days). It is expected that because of severe global climate change, the level of the two indicators as mentioned above will increase in the future (19).
Zoonotic infectious diseases
Despite evidence of the impact of climate change on infections (20, 21), its effect on zoonotic diseases has been largely unknown. Zoonotic diseases are in general, characterized as infections transmitted from animal to human (and less frequently vice versa), either indirectly (via an intermediate vector such as an insect or an arthropod) or directly (via contact with animal products) (22). Many zoonotic diseases are prone to increase because of shifts in the distribution and behavior of animals and vector species, demonstrating that biological systems are now adjusting to ecological variations (23). In this regard, climate variability may expand the current limits of agricultural activities, increasing the opportunity for contact between species that have not regularly interacted in that area. Animals excrete many microorganisms, including pathogens, that can be transmitted to humans through food and water. The risk of transmission to humans increases if food crops are watered with contaminated water (24, 25). Densely crowded urban environments, particularly those without sufficient sanitation, are significant public health concerns as they can be sources of disease epidemics. Despite the scientific and public health interest and opportunities for these diseases, they have yet to receive corresponding attention.
These diseases and other zoonotic diseases address a significant disease burden, from lower endemic areas to those from highly endemic areas (23). These diverse epidemiological situations have endured the effects of climate change in the socio-economic systems, such as fishing and agriculture, as a result of the phases of the El Niño Southern Oscillation (ENSO) phenomena, but also in specific health conditions such as infectious, zoonotic and tropical diseases; such as Chagas disease, brucellosis, leishmaniasis, toxocariasis, among others (23). The list of zoonoses currently studied for the effect of climate variability includes cholera (26), leptospirosis (27), anthrax (28), yellow fever (29), hantavirus infections (30), rabies (31), schistosomiasis (32), leishmaniasis (33), babesiosis (34), giardiasis (35), among others (36). This section will provide an overview of crucial bacterial zoonotic diseases.
Leptospirosis
Leptospirosis is a waterborne zoonotic acute febrile illness caused by spirochetes of the genus Leptospira spp., an emerging and reemerging bacterial illness in some regions of the world (37). Leptospirosis is known as an emerging infection that is sensitive to climate change. Climate variability can affect its transmission and occurrence. Floods and prolonged rainfall have been associated with outbreak of leptospirosis throughout the world, and climate change has potentially led to an increase in the disease’s prevalence and the magnitude of its outbreak. Studies have shown that it infects over 180 animal species, including domestic and wild animals such as pigs, horses, cattle, dogs, mice, and other rodents. Outbreaks of leptospirosis have been documented in different parts of the world, including Southeast Asia, Nicaragua, Brazil, Malaysia, India and the USA These bacteria are caused by speedy, unplanned urbanization and weak sanitation, making leptospirosis a major cause of acute febrile illness in developing countries. With speedy, universal urbanization, close to 68% of the world’s human population is planned to be living in urban areas by the year 2050 (38). One animal that benefit from urban life is wild mice, which have been seen growing in urban and domestic environments, including large urban populations particularly in India, Brazil, and Tokyo (39), leading to frequent human contact with these species (40). Other factors that increase these bacteria following climate change include upping sea flats, upping sea and land shallow temperatures, increased frequency of severe weather events, more severe tropical storms, and more storm surges (41). Because this bacterium can survive in humid environments for a long time, floodwater provides ideal conditions for its survival (42). A study reported that following a flood, Leptospira spp. accounted for 45% of the pathogens found to permeate water supplies (43). There are other studies in South Asia and Central and South America on the prevalence of flood-associated leptospirosis (Will's disease) (44-46). The disease is problematic, especially for the suburban population in low-income countries, including a flood of open sewers and streets (47). Besides floods and rainfall, there is an increase in temperature for transferring leptospirosis. Predicted global temperature increase of 1.4–5.8 °C the year 2100 are associated with an increase in leptospirosis. Therefore, global warming can prolong the seasons and expand geographical areas for optimal survival (48). It is predicted that the annual quantitative relationship of infection varies from 0.02 per 100,000 in temperate climates to 10 to 100 per 100,000 in tropical environmental conditions, but outbreaks may occure anywhere, including in slums of the developing world. About 1.03 million cases of human leptospirosis appear globally, resulting in 2.9 million critical conditions focused on life years lost (49). A society's vulnerability to climate variation- induced health hazards of infectious diseases is depended on its social expansion. In countries with vulnerable infrastructure, poor sanitation and lack of clean drinking water, the risk of spreading the this disease through water increases, especially following floods and outbreaks (43).
Anthrax
Zoonotic diseases have significant importance in both animal and public health. In this regard, some new research have emphasized the role of climate change in these types of disease transmission (50). One of the most harmful and lethal zoonosis is Bacillus anthracis which is transmitted to human mainly through contact with animal products (mainly cattle), spores, and infected animals, but anthrax transmission is possible through both mosquitos and flies (51, 52). B. anthracis is sensitive to soil moisture, acidity and organic content, and its life cycle is influenced by climatic factors uch as ambient temperature and rainfall (53). Therefore, some areas can be affected by this disease more than others. In this regard, in endemic areas of anthrax, high incidence occurs in dry and hot periods after heavy rains, which explains the major outbreaks of diseases in countries with hot climates such as Ethiopia and South Africa (53). It is worth mentioning that the weather has an effect on the high resistance of spores against adverse conditions and their ability to reproduce. According to the ‘anthrax belts’ production by some environmental conditions, the soil becomes highly contaminated with B. anthracis spores (54). Some papers indicated the prerequisites for this contamination such as soil with pH > 6.0 and fast change of weather from rainfall to dry conditions (51). However,the high ability of the B. anthracis to survive in the environment for decades allows them to resist extremes PH and temperatures (51). Since permafrost thawing exposes carcasses buried in ice and subsequently flooding and soil disruption, especially in the Russian Arctic, this phenomenon has played a significant role in releasing thawed anthrax spores (54). Based on the results of some papers, the high temperature of 2016 in Yamal (6.7 °C higher than the usual mean in June), low levels of air humidity (less than 30%) resulting from the minimal rainfall, excessive land use and finally lack of sufficient preventive policies like animal vaccination led to high outbreaks of anthrax (52, 55, 56).
Since climate change has shown a sufficient ability to determine population density, niche of livestock and human, infected human host convergence, and transmission season, its effects on the timing and persistence of an anthrax outbreak (57). The highest record of warming around the globe was in 2001-2010 and occurring considerable precipitation in the Livno region led to massive flooding, water evaporation, and spore concentration that accelerate anthrax transmission (54, 57). In addition, it was reported that there was an association between spring time flooding followed by a dry summer and anthrax outbreaks in Sweden and Bosnia and Herzegovina (B&H). Since climate change shows a significant effect on terrestrial ecosystem structure, vegetation covers, soil composition, and the distribution of wild ruminant species, warming temperatures and dry conditions may enhance the transmission of B. anthracis (54).
As long as numerous reports about the effects of the climate change on the anthrax outbreak, at present we know that some factors such as interventions targeting animal health promotion among livestock herds (like high animal vaccination), minimal land use, and normal summer temperatures may mitigate further outbreaks effects of climate changes on anthrax transmission (52, 54).
Brucellosis
Human brucellosis is a significant public health theme in the universal livestock husbandry region (58). It is a bacterial disease of a multitude of hoofed animals and carnivores, which causes a broad diversity of symptoms in humans and the disease distribution is mainly associated with climate mutability (59). Many studies have associated the most important causes of human brucellosis infection with animal locale, job, host denseness, socioeconomic position, movement, and in-migration (60, 61). Also, the statistical distribution of human brucellosis in other studies supports that cases are regularly clustered in occupational and food-associated positions (62). In many geographical regions, there is a significant deficiency of reports of animal and human brucellosis, because of limited access to advanced identification methods and in the community pieces of information on, pieces of evidence, and caring of the illness, especially in many countries of Africa and South America (63, 64). They still ignore investigations of this zoonotic in many geographical regions of these countries. Ecological factors in perspective, it is very important factors that can cause brucellosis. For example, the most general reason for human brucellosis, Brucella melitensis (animal hosts: camels, goats, sheep); Brucella abortus (animal hosts: camels, buffalo, elk, cattle, yaks,), the second most common origination of human infection. It has also been enclosed human infections after cases accorded, Brucella. canis, Brucella. ceti, Brucella. suis, and Brucella. Nominate (64). In recent years, according to statistics, human brucellosis has been re-emergent in China and the definite quantity of human brucellosis arrived at a historic high-top in 2009. At this historical moment, it has been observed that men had the highest prevalence of the disease in the age group of 30 to 50 years. It relates the reason for this to the livestock occupation, in which men are more economically active (65, 66). Some reports have indicated that the seasonality of brucellosis can be related to ecological factors, especially climate variability. Terms of geographical studies of lowland areas and moderate elevation, which are suitable habitats for sheep and goats, have seen more risk factors for brucellosis in humans. Of course, it has also been seen some exceptional areas, including the place of Qinghai, Tibet and western Sichuan in the Qinghai-Tibet plateau that has major areas of grassland of moderate rise but a very low-toned comparative relative frequency of human brucellosis (0.78/100,000) (67). Currently, the area-to-area shifting of brucellosis may link some endemic regions to ecological agents and variations in management patterns (68). In general, common diseases between humans and animals show a significant burden of diseases, from very native to low endemic areas (69, 70). Microbiological experiments have shown that Brucella strains grow optimally at 37 °C for 48 hours (71). The results of recent studies show that temperature changes are a factor that has a direct impact on the human brucellosis epidemic compared to other climatic variables. The raising temperature increased Brucella spp. extension and replication in the host and increased the frequency of showing of susceptible human and animals (72). Thus, the transmission and persistence of Brucella spp. may increase in warmer conditions. Rising temperatures in late spring and early summer increase sheep and goat breeding activities, including breeding, shearing, meat processing, and commercialization of sheep products, resulting in increased exposure to animals susceptible to contaminated animal products (73). In mutuality, the results show that the transmission of brucellosis decreases in winter because colder temperatures may severely limit the growth of infectious organisms (74). The mutability in monthly stockpiled evaporation and sunshine impresses the transition of Brucella spp., which expands intracellularly in the host and without the host in the water, dust, and soil (75).
Vector-borne disease
Three components of a host (or vector), an agent (or pathogen), and a transmission environment are essential for most infectious diseases (76). A few pathogens require intermediate hosts or conveyed by vectors to complete their lifecycle. Lately, a few vector-borne diseases have (re)-emerged and spread worldwide, because of local and/or global changes that have prompted the introduction and establishment of new pathogens, the improvement of their vectorial capacity, or the invasion of new arthropod vectors (77). Indeed, worldwide climate change will influence disease vector behavior, which in turn may alter the current patterns of vector-borne diseases transmitted by the bite of hematophagous arthropods (78). This is of importance in assessing for instance the right planning for preventive treatments against feline and canine heartworm disease, which must ensure protection from infection during the entire risk season. In addition to climatic changes, other main factors for the (re)-emergence and spread of vector-borne parasites include globalization and the increase in international trade, pollution, alterations in water storage and irrigation habits, habitat changes, development of insecticide and drug resistance, tourism and travel (79). Some of the vector-borne diseases that might be influenced by climate changes and the above-mentioned drivers include Lyme disease, plague, ehrlichiosis, malaria, Chikungunya infection, yellow and dengue fever, bluetongue, encephalitis, leishmaniosis, West Nile disease, trypanosomosis, and dirofilariosis (80).
Lyme disease and Tick-borne infections
Reports on the effects of climate change on ticks and tick-borne infections are generally scarce, mainly because in some parts of the world, long-term findings on the distribution and abundance of tick populations and the prevalence and occurrence of tick-borne infections are not exist. However, some evidence has noted that climate change has helped expand the range of ticks, increasing the potential risk of Lyme disease, where ticks were previously unable to survive. In this regard, the life cycle and prevalence of the deer tick is strongly influenced by temperature (81). The main cause of Borreliosis (Lyme disease) is Borrelia burgdorferi. The two main species of tick (Ixodes scapularis and Ixodes pacificus) can transmit Borrelia burgdorferi to humans (82) Borreliosis normally illustrates an erythema migrans rash and non-specific symptoms. The prevalence of erythema migrans is associated with some environmental factors, for example, summer temperatures, average rainfall and the number of hot days with humidity above 86%, and the number of winter days with temperatures below zero degrees Celsius. Therefore, the risk of infections due to the frequency of ticks seems to depend on environmental factors (83).
Tick-borne borreliosis (TBB), similar to other tick-borne diseases, is one of the diseases whose cycle and distribution are significantly affected by temperature and rainfall. Warmer temperatures, through the production and propagation of ticks, increase woodlands and provide favorable conditions for the spread of ticks. On the other hand, increasing rainfall and consequent severe flooding can reduce the population and types of ticks. Of course, it should not be overlooked that socio-economic factors can also affect the rate of exposure to ticks and subsequent tick-borne diseases. Even if we are optimistic about a climate change scenario in which there is only a 1.5° C rise in temperature, Lyme disease can still spread to northern Canada in the future. As a result, there is overwhelming evidence that global warming could increase the number, type, activity level, and geographic distribution of ticks in North America and directly lead to the spread of black-legged ticks and Lyme disease in Canada (52, 82). A study also showed that mild winters and hot, humid summers could increase the incidence of Borreliosis in southern Sweden (83).
Due to the prolonged overwintering of ticks, the worldwide warming facilitates the migration of tick-borne encephalitis and Lyme disease to the poleward. Early springs and mild winters in Sweden have been reported to have an important effect on increasing tick-borne encephalitis. It has also been reported that a 20-degree increase in California's average temperature is related to a doubling of the transmission season. Also, the amount of encephalitides has a positive relationship with heavy rain, which is usually more common in the context of climate change (84). Due to the expected increase in global warming, the vector range of I. ricinusis is predicted to expand considerably to the north and east, where Tick-borne encephalitis (TBE) and borreliosis are currently rare (52, 82, 85). Another study in Sweden found that warming not only increased TBE but also increased the incidence of other diseases transmitted by I. ricinus, such as human ehrlichiosis and Lyme borreliosis (86). In addition to the above, other pathogens are sensitive to climate change, such as Crimean-Congo hemorrhagic fever, babesiosis, and rickettsioses. Described patterns of tick-borne diseases such as an altitudinal and latitudinal transmission of ticks have been reported at various temperatures and in the peri-Arctic areas of the northern hemisphere over the past decade. This warns of the direct effect of climate change factors on Ixodes tick habitats (82).
Although other tick-borne diseases such as granulocytic anaplasmosis and human babesiosis have emerged and spread in North America and Eurasia as the temperature warms, it must be said that it is difficult to separate the negative impacts of climate change from other harmful potential factors (87). Anaplasmosis originates mainly from Anaplasma phagocytophilum, which is often spread by I. scapularis in central and eastern Canada (85).
Tularemia
It has been well documented that climate change factors (temperature, precipitation and humidity) is able to explain at least a significant part of disease transmission such as tularemia (52). According to its close relationship with local vectors, specialists believed that tularemia is frequently sensitive to environmental change (88). The bacterium Francisella tularensis belongs to the facultative intracellular pathogens and is the most important cause of tularemia, divided into 4 subspecies. The most virulent subspecies are tularensis (type A) and holarctica (type B), which are prevalent in North America and Europe, respectively. Transmission factors to tularemia are almost widespread. Scientists consider some routes for this disease: consumption of contaminated meat or water, direct contact with infected rodents, hares, and arthropod vectors (mainly ticks, and mosquitoes), and exposure to a contaminated soil environment (52, 83). Tularemia is prevalent in the Northern hemisphere (especially Sweden, Finland, and Turkey) and generally led to seasonal outbreaks (83).
A study on the Swedish cases demonstrated that there was a link between tularemia with some factors like the high number of cases in the previous year, mosquito abundance, high precipitation, and finally high temperatures. It should be noted, however, that due to the lack of consideration of human factors in this study, it seems that there is some overestimation of the role of mosquitoes in disease transmission (88). According to some studies, the prevalence of the disease in Sweden has increased about 10 times in the last thirty years, mainly through mosquitoes and frequently during August and September (89). Another study shows that the concentration of tularemia in Sweden is located in a few high-risk regions with high seasonal and annual variations (88). In the USA, the incidence of tularemia was positively associated with coverage by dry and forested habitats suitable for ticks in Missouri, Kansas, Arkansas, Oklahoma, and Nebraska but not Tennessee, Indiana, Illinois, and Kentucky (90).
Francisella tularensis might reside in major river watersheds or a water-dwelling unicellular eukaryote. In this regard, there are extensive studies and various epidemics (Kazakhstan, Turkey, Spain, and the Soviet :union:) that confirm the association between tularemia and natural waters (52, 91-93). According to the association between tularemia and natural waters, some climate factors (cold winter with low precipitation, existing runoffs in the watershed area) decreasing the amount of circulation in water bodies, can cause bacterial density and growth of protozoans to spread to arthropod vector populations such as mosquitos and subsequently humans. Because the relationship between tularemia and cold weather is positive, it cannot be expected that climate change with high temperatures would facilitate the prevalence of tularemia (94). In contrast, some studies believed that there is a reverse relationship between the population of F. tularensis and little snow cover. According to the data, the previous summer temperature, possibly, is a significant factor in the size of the F. tularensis population in nature (95). Furthermore, it is estimated that a change in environmental factors like antecedent drought may reduce competitor species of mosquito larvae or excessive rainfall can decimate some mosquito populations by flushing larval habitats, prompting elevated rates of mosquito production (96).
For treatment, it should be said that despite the effectiveness of the tularemia vaccine and due to financial constraints, the level of vaccination in the affected areas has been low. Of course, the ineffectiveness of prevention in some regions can be related to the hydrographical features (95). The fact is that due to the sometimes-contradictory results on how tularemia is affected by climate change, of course, more extensive studies are needed internationally. These studies can provide more accurate models to identify specific preconditions for the disease, leading to introducing more effective treatments for tularemia (96).
Vibrio spp. Infections
Temperate marine areas have been severely affected. For example, Europe's seas have warmed four to seven times over the past few decades (97). Climate change has been noted a dramatic impact on marine fauna and flora communities, but little is known about its impact on marine microorganisms, which represent the largest living biomass in the world's oceans and are essential for sustaining life on the planet. The link between infections caused by worldwide warming and waterborne pathogens is considerably intriguing. A rise in sea surface temperature in a given region occurring shortly before an increase in the number of individuals infected with cholera has been recommended as a potential effect of climate change on disease spreading (98). In 1970, primary Vibrio cholerae outbreaks began outspreading amongst the mainland, with epidemics reported in Africa (Guinea) and the Horn of Africa (Ethiopia, Somalia, and Sudan). The severe cholera outbreak on the African mainland was in 1998, accounting for over 72% of the worldwide whole number of cholera instances (99, 100). This genus entails over 100 characterized species, and it has shown a dozen species to cause infections in humans (101). Infection with these bacteria mostly starts from exposure to seawater (in developing countries) or utilization of raw seafood (in the developed world) and affects millions of people annually with a wide mortality rate (100, 102). These bacteria prefer to adhere self to the surface of chitinaceous zooplankton and shellfish (103). Zooplankton and shellfish increment in numbers, following major bursts of phytoplankton related to warm sea surface temperatures (104, 105). It associates coastal Vibrio infections with zooplankton blooms, warmer water, and intense storms (106).
Some waterborne infections by pathogenic Vibrio spp., poleward extension correlates with augmented universal temperature and lower salinity of aquatic environments in riparian regions affected by increased precipitation (106). This change in temperature conditions can cause the upward growth of Vibrio spp. in the environment (97). In humans, it has been linked to exposure to diarrheal diseases to warmer temperatures and many rainfalls (107).
Climatic change and enteric diseases
Enteric infections caused by bacterial and viral pathogens affect the regular functioning of the intestinal tract, resulting in several gastrointestinal manifestations such as nausea, vomiting, and diarrhea (108). Although the burden of enteric infection remains high, significant reductions have been achieved, especially in mortality. However, progress in reducing the number of enteric infections in the future could be affected by climate change, particularly as ambient temperatures rise (108). The effect of ambient temperature on the occurrence of some enteric diseases has been shown in different examinations. The temperature could be the key climatic marker in the transmission of bacillary dysentery. It was showed that a potential 1°C increase in temperature is associated with up to 16% increase in bacillary dysentery cases in the southern city and a 12% increase in the northern city of China (7). This outcome is in line with other studies on the effects of temperature on enteric infections. In Peruvian children, for example, each 1°C increase in temperature was associated with an 8% increase in the risk of severe diarrhea (109).
Similar to many foodborne pathogens, seasonality as a defining impact on disease incidence has been considered for human campylobacteriosis and salmonellosis (110), emphasizing the importance of public health in seasonal outbreak patterns. However, the researchers do not demonstrate unambiguously seasonality, as geographical, climatic, or production- associated factors prompt various outcomes. The seasonality of campylobacteriosis, if present, correlates to the seasonality of campylobacter colonization in commercial broiler flocks (111). A 1°C rise in temperature has been reported to correspond to a 5% increase in the number of campylobacteriosis reported cases in England (112). For Salmonella, the link between the occurrence of human salmonellosis and seasons is regularly clarified by gardening or barbecuing, not by the contamination of chickens (113), but also shedding by other livestock such as cattle and pigs should be considered. These examinations suggest that variables associated only with dietary choices and behavior are unlikely to clarify all of the seasonal summer increases in certain bacterial gastroenteritis noted in temperate latitudes (114). Warmer ambient temperatures in combination with differences in eating behavior may therefore contribute to the foodborne portion of the increased incidence of enteric diseases (115).
Climate change and antibiotic resistance
In the current and leading decades, climate change and antimicrobial resistance have become two serious problems of humanity, and reducing the use of fossil fuels and antibiotics seems necessary to manage these problems, and policies based on these principles for behavioral changes is essential. One of the essential pillars that keeps our current healthcare system functioning is the availability of effective antibiotics for bacterial infections (116). Without effective antibiotics, surgeries, cancer treatments, organ transplants, and community-acquired infections can be fatal, claiming millions more lives each year. In addition, some of the gains in childhood survival are being lost due to the availability of effective antibiotics for respiratory infections (116). With climate change, this situation is getting closer to the tipping point because climate change and antibiotic resistance are closely linked. The temperature has long been known to influence bacterial growth in-vitro as well as modulate the transfer of genomic material, including genes encoding (or conferring) antibiotic resistance (117, 118), but there is limited evidence on the population level of resistance and the effect of climate variability. Assessing the association between antibiotic resistance of the three most prevalent pathogens (S. aureus, Klebsiella pneumoniae, and Escherichia coli) and temperature, McFadden et al. (119) found that for every 10° C increase in minimum temperature, the resistance rate was increased as 2.7%, 2.2%, and 4.2%, respectively. The result of McGough et al. (120) paper was also consistent with McFadden; they examined the effects of temperature on resistance in 28 European countries from 2000 to 2016 and concluded that not only there was a correlation between warmer temperatures and the overall presence of antibiotic-resistant bacteria, but also, they found that there is a relationship between warmer temperatures and the high levels of similar antibiotic-resistant bacteria (120). More importantly, they found that even after controlling for known factors such as overall antibiotic use and human population density, antibiotic resistance still increases.
It should be noted that a detailed study of the observed relationships between antibiotic resistance and air temperature requires further researche. However, there are some hypotheses in this regard. First, higher temperatures, through the exchange of resistant genes, may facilitate horizontal gene transfer [e.g., Extended-spectrum beta-lactamases (ESBLs)] or the uptake of genetic materials. European countries in the southern latitudes show a high prevalence of ESBL-related infections, which naturally play a role in the use of antibiotics and are facilitated by climatic elements such as temperature. Second, temperature is one of the strongest determinants of bacterial growth and may determine the carriage and transmission of bacterial species between humans and animals (119).
Climate change-related migration and infectious disease
The results show that out of its detrimental effect on the environment, crowd development is a remarkable reason for enhancement in the number of environmental emigrants. Because of continued migration, the United Nations estimates that by 2025, about 65% of the world's population, including 61% of the population in developing areas, will live in urban areas (121). As cities and urbanization increase, other environments shrink over time. This issue of population increase and global warming will cause sea levels to rise and encroach on large areas of the earth, this will be a very serious and dangerous problem for the people (122). The migration of people and even animals take them to an unfamiliar environment where these changes may not be familiar to the local pathogens. In Kenya, for example, investigations show that Masai herders lose many animals because of East Coast fever and suffer financial losses when moving during drought seasons to higher elevations (in the foothills of Mount Kenya (42).
In general, population movements can take place within a country, or in the form of international fluxes. Unpredictable human migration can put pressure on unprepared health infrastructure, particularly in the face of new and emerging indigenous diseases of the migrant and resident population in the region. It is estimated that human migration has been an important source of epidemics in human history and has undergone fundamental changes in the climate, leading to the spread of many infectious diseases such as smallpox and plague. Unexpected and massive population migration also causes limited access to health and medical services, lack of drinking and food water, mixed populations, and inefficient barriers to transporters and animals (123). We have observed another example that immunocompromised population such as human immunodeficiency viruses (HIV) patients may also be more prone to increased pathogen loads associated with higher temperatures and environmental changes, while factors such as poor hygiene have already impaired their resilience (124).
The pandemic caused by the emergence of Coronavirus disease 2019 (COVID-19) demonstrated the significant vulnerability of humans to infectious diseases. Such diseases can not only cause injury and death in a large number of people but can also bring negative socio-economic results. However, there is increasing scientific interest in the potential effects of global climate change on human health. The impact of climate change on public health can be through the impact on pathogens, hosts, vectors, and disease transmission. Although numerous investigations actually may have some limitations; such as the lack of incorporation of other meteorological components into the analysis, it has been proposed that such discoveries are important, from a public health perspective, to better understand the ecoepidemiology of different diseases. Nonetheless, more examination is required in different parts of the world to develop monitoring systems that will help in predicting the effect of climate changes on the occurrence of these diseases in endemic regions with different social and biological conditions.
Not applicable.
Not-Required.
Conflicts of Interest
None of the authors have any conflict of interest.
Received: 2023/02/7 | Accepted: 2023/04/19 | ePublished: 2023/06/26
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |