BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1931-en.html
2- Department of Biotechnology, Animal Health Research Institute, Agricultural Research Center (ARC), Reference Laboratory for Veterinary Quality Control on Poultry Production, Giza, Egypt
3- Department of Food Hygiene, Animal Health Research Institute, Agricultural Research Center (ARC), Giza, Egypt
As a result of their high moisture content, plenty of nitrogenous compounds (amino acids, peptides, and proteins), and availability of minerals and auxiliary growth agents, meat and meat products are ideal for a variety of species to flourish. They also have a small amount of fermentable carbohydrates, usually in the form of glycogen, and they keep their pH at a level that encourages the growth of the majority of bacteria (1).
A Gram-positive, rod-shaped endospore-forming bacterium called B. cereus is a major contributor to food-borne disease in people and is regularly engaged in food outbreaks (2).
It's possible for contamination or intoxication to cause B. cereus-related nutrition damage. Spores that develop in the small digestive tract and release diarrheal enterotoxins are contaminated when consumed (3).
Bacillus cereus is a rod-shaped, Gram-positive, motile, facultatively anaerobic, aerobic, and spore-forming bacterium that is frequently encountered in the environment or as food contamination. The model organism of the Bacillus cereus genus, commonly known as B. cereus sensu lato, is Bacillus cereus sensu stricto (hereinafter B. cereus). This complex has up to ten heredities of species closely associated with hereditary and phenotypic (4).
Despite being fairly salt-resistant and able to multiply at pH levels between 5 and 10, Bacillus cereus vegetative cells are rapidly destroyed by thermal treatments like pasteurization or frying (5). On the other hand, spores are extremely resilient to harsh circumstances, including tall temperatures, solidifying, drying, and gamma- and UV-irradiation, empowering B. cereus to outlive within the environment and on shifted surfaces.
The B. cereus group contains a large number of phylogenetically similar species, like B. cereus, B. anthracis, B. thuringiensis, B. cytotoxicus, and others.B. toyonensis, B. mycoides, and B. pseudomycoides (6).
The B. cereus manufactures enterotoxins because hemolysin BL (Hbl), nonhemolytic enterotoxin (Nhe) complex, and cytotoxin K (cytK) proteins are thought to be the main enterotoxin causes in B. cereus diarrhea (7). Also, the emetic toxin can be formed by B. cereus (8). The molecular methods can predict the existence or absence of the target enterotoxin gene. The analysts predicted that the utilization of atomic strategies permits the discovery of B. cereus food poisoning gene targets than strain assurance (9). The work aimed to replace routine culture testing, used to investigate food products that may have been contaminated with Bacillus cereus, by using PCR to directly detect the bacteria and some of its enterotoxins from food.
2-1- Collection of samples
A total of 75 random samples of Kibda sandwiches, Sausage sandwiches, Chicken Luncheon, Beef luncheon meat, and Chicken Shawarma (Fifteen samples of each) ) were collected from distinctive places in numerous regions in Kafr El-Sheikh governorate, Egypt. The samples were put in isolated, sterile packs and exchanged straightforwardly to the laboratory for assisted bacteriological examination.
2-2- Preparation of samples
Based on (10), the samples were prepared for bacteriological examination. Specifically, one portion of the food samples was primarily cultivated on Trypticase Soy Broth (TSB) at 37˚C for 24 hours, after which the broth samples were subjected to traditional isolation, identification of bacteria by biochemical test, and PCR, which was represented by group A. The other portion of the food samples was processed for extracting DNA from samples directly for rapid detection of Bacillus cereus & its enterotoxins (group B which represents samples taken from food related to positive isolates, and group C taken randomly from food of negative isolation results).
2-3- Bacteriological examinations:
Isolation of Bacillus cereus
Trypticase Soy Broth (TSB) tubes were filled with the necessary quantity of each prepared sample and subsequently incubated for 48 ± 2 h at 30 ± 2 ◦C. then, a loop of the produced cultures was streaked on mannitol egg yolk polymyxin agar plates (MYP), which were incubated 48 ± 2 h at 30 ± 2 ◦C. Pink colonies encircled by a zone of precipitation and suspected to be lecithinase-positive colonies were chosen for morphological and biochemical identification.
Identification of bacterial isolates
The separated microscopic organisms were recognized as infinitesimally and biochemically concurring (11). The distinguished B. cereus strains were motile Gram-positive large rods with non-swelling spores, lecithinase positive and on MYP agar, β-hemolytic on blood agar. Biochemically, all isolates passed the Voges-Proskauer, nitrate reduction, catalase, citrate utilization, and glucose fermentation assays. They failed the tests for methyl red, indole, mannitol fermentation, H2S generation, and oxidase. Detection of groEL targeted primer for detection of B. cereus & some enterotoxins genes using Polymerase Chain Reaction (PCR).
Extraction of DNA.
The QIAamp DNA Smaller than expected unit (Qiagen, Germany, GmbH) was utilized to extricate DNA from tests, with a few alterations made to the manufacturer's information. In a nutshell, 200 µL of the test suspension was treated at 56 OC for 10 min with 10 µL of proteinase K and 200 µL of lysis arrangement. 200 µL of 100% ethanol was included in the lysate after brooding. After that, the test was cleaned and centrifuged after the manufacturer's enlightening. The kit's 100 µL of elution buffer was utilized to elute the nucleic corrosive.
Oligonucleotide Primer
The preliminaries utilized were provided from Metabion (Germany) and are recorded within the table (1).
PCR amplification
PCR A 25 µL response containing 12.5 µL of EmeraldAmp Max PCR Ace Blend (Takara, Japan), 1 µL of each preliminary at a concentration of 20 pmol, 5.5 µL of water, and 5 µL of DNA format was utilized to test the preliminaries. Warm cycle 2720 from Connected Biosystems was utilized to carry out the method.
Analysis of the PCR Products.
The PCR items were isolated utilizing 5V/cm angle electrophoresis on a 1.5% agarose gel (Applichem, Germany, GmbH) in 1x TBE buffer at room temperature. Each gel space had 20 µL of the products embedded for the gel examination. The sizes of the parts were decided to employ a 100 bp Generuler step from Fermentas in Germany. A gel documentation framework (Alpha Innotech, Biometra) took pictures of the gel, and the computer program was utilized to analyze the information. Details of the groundworks utilized were recorded in (Table 1).
| Target gene B. cereus |
Primers sequences | Amplified segment (bp) | Primary denaturation |
Amplification (35 cycles) | Final extension | Reference | ||
| Secondary denaturation | Annealing | Extension | ||||||
| groEL | TGCAACTGTATTAGCACAAGC T | 533 | 94˚C 5 min. |
94˚C 30 sec. |
55˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
(12) |
| TACCACGAAGTTTGTTCACTACT | ||||||||
| nhe | AAG CIG CTC TTC GIA TTC | 766 | 94˚C 5 min. |
94˚C 30 sec. |
49˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
(13) |
| ITI GTT GAA ATA AGC TGT GG | ||||||||
| cytK | ACA GAT ATC GGI CAA AAT GC | 421 | 94˚C 5 min. |
94˚C 30 sec. |
49˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
|
| CAA GTI ACT TGA CCI GTT GC | ||||||||
3-1- Incidence of Bacillus cereus.
Table 2. Incidence of Bacillus cereus isolated from examining Kibda sandwiches, Sausage sandwiches, Chicken luncheon, Meat luncheon and Chicken shawerma sandwiches.
| Types of Samples | No. of samples | No. of positive samples | Percentage % |
| Kibda sandwiches | 15 | 6 | 40 |
| Sausage sandwiches | 15 | 4 | 26.6 |
| Chicken luncheon | 15 | 3 | 20 |
| meat Luncheon | 15 | 2 | 13.3 |
| Chicken shawerma sandwiches | 15 | 2 | 13.3 |
| Total | 75 | 17 | 22.6 |
Molecular characterization of groEL gene of Bacillus cereus group & some enterotoxins genes (nhe & cytK) of Bacillus cereus.
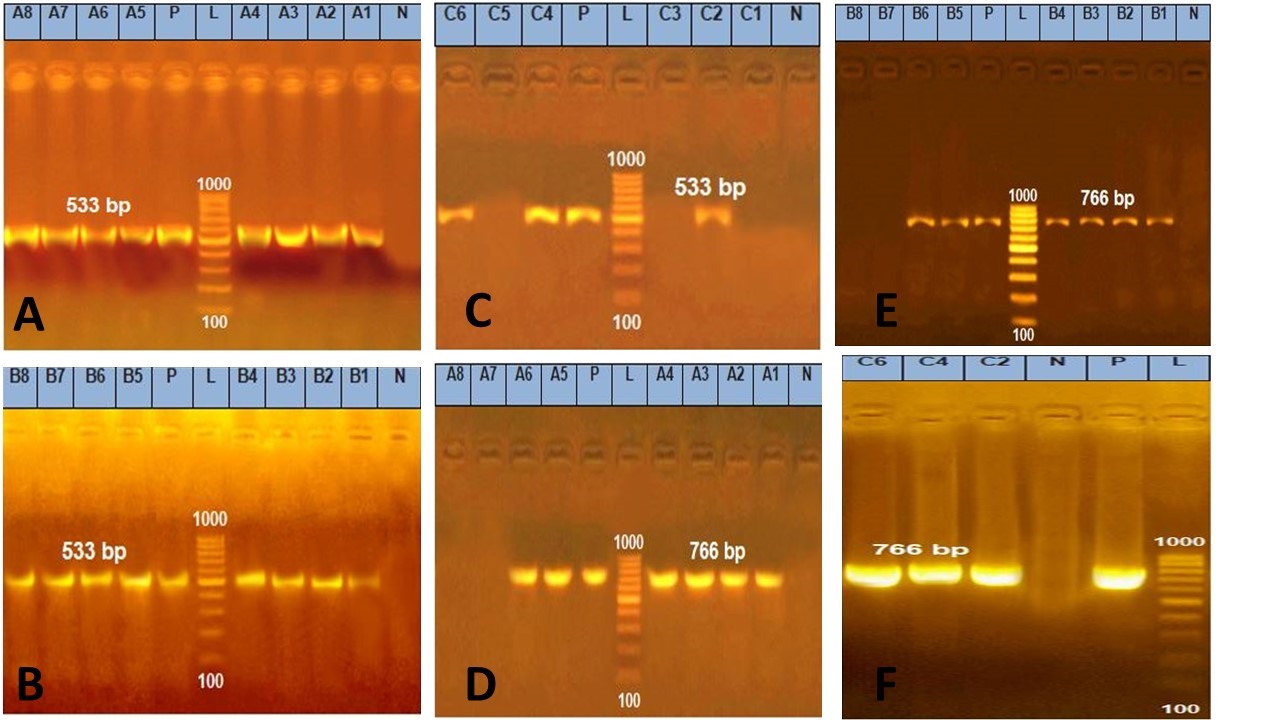
Twenty two samples divided into three groups (group A represents 8isolates, group B represents 8samples taken directly from food related to isolates and group C representative 6samples taken directly from food of negative isolation results) subjected to molecular characterization of groEL gene of Bacillus cereus & enterotoxins genes (nhe & cytK). Our results cleared that all isolates of group A & samples taken directly from food related to isolates (group B) carried groEL gene by 100%, group C carried groEL gene by 50%, while the enterotoxins genes nhe& cytK were presented by 75% & 25% in group A & group B respectively, but in the group C were represented by 50% for each (Table 3) (Figure 1 and 2).
Table 3. Prevalence of groEL gene for detection of Bacillus cereus in the three groups and some of its enterotoxins genes (nhe &cytK)
| GP | Sample | groEL | nhe | cytK |
| A (isolates) | A1 | + | + | - |
| A2 | + | + | - | |
| A3 | + | + | - | |
| A4 | + | + | - | |
| A5 | + | + | - | |
| A6 | + | + | - | |
| A7 | + | - | + | |
| A8 | + | - | + | |
| Total (%) | 8 (100%) | 6 (75%) | 2 (25%) | |
| B (Food related to isolates) | B1 | + | + | - |
| B2 | + | + | - | |
| B3 | + | + | - | |
| B4 | + | + | - | |
| B5 | + | + | - | |
| B6 | + | + | - | |
| B7 | + | - | + | |
| B8 | + | - | + | |
| Total (%) | 8 (100%) | 6 (75%) | 2 (25%) | |
| C (Food of negative isolation result) | C1 | - | ND | ND |
| C2 | + | + | + | |
| C3 | - | ND | ND | |
| C4 | + | + | + | |
| C5 | - | ND | ND | |
| C6 | + | + | + | |
| Total (%) | 3 (50%) | 3 (50%) | 3 (50%) |
Figure 1. Agarose gel electrophoresis of PCR amplification products of groEL and nhe enterotoxin genes
-
Amplification of 533 bp of groEL gene among group A. Lane L: 100-1000 bp DNA molecular size marker , Lane P: Control positive Bacillus cereus groEL gene 533bp , and Lanes 1, 2, 3, 4, 5, 6, 7 and 8: Positive Bacillus cereus isolates.
-
Amplification of 533 bp of groEL gene among group B. Lane L:100-1000 bp DNA molecular size marker , Lane P: Control positive Bacillus cereus groEL gene 533bp, and Lanes 1, 2, 3, 4, 5, 6, 7and 8: Positive Bacillus cereus samples for groEL gene.
-
Amplification of 533 bp of groEL gene amonggroup C. Lane L:100-1000 bp DNA molecular size marker, Lane : Control positive Bacillus cereus groEL gene 533bp, and Lanes 2, 4 and 6: Positive Bacillus cereus samples for groEL gene)
-
Amplification of 766 bp products of nhe enterotoxin gene among group A. Lane L:100-1000 bp DNA molecular size marker, Lane P: Control positive Bacillus cereus nhe enterotoxin gene at 766bpand Lanes 1, 2, 3, 4, 5 and 6: Positive Bacillus cereus isolates for nhe gene.
-
Amplification 766 bp products of nhe enterotoxin gene among group B. Lane L: 100-1000 bp DNA molecular size marker, Lane P: Control positive Bacillus cereus nhe enterotoxin gene at 766bp and Lanes 1, 2, 3, 4, 5and 6: Positive Bacillus cereus samples for nhe gene.
-
Amplification products of 766 bp nhe enterotoxin gene among group C. Lane L:100-1000 bp DNA molecular size marker, Lane P: Control positive Bacillus cereus nhe enterotoxin gene at 766 bp and Lanes 2, 4and 6: Positive Bacillus cereus samples for nhe gene.
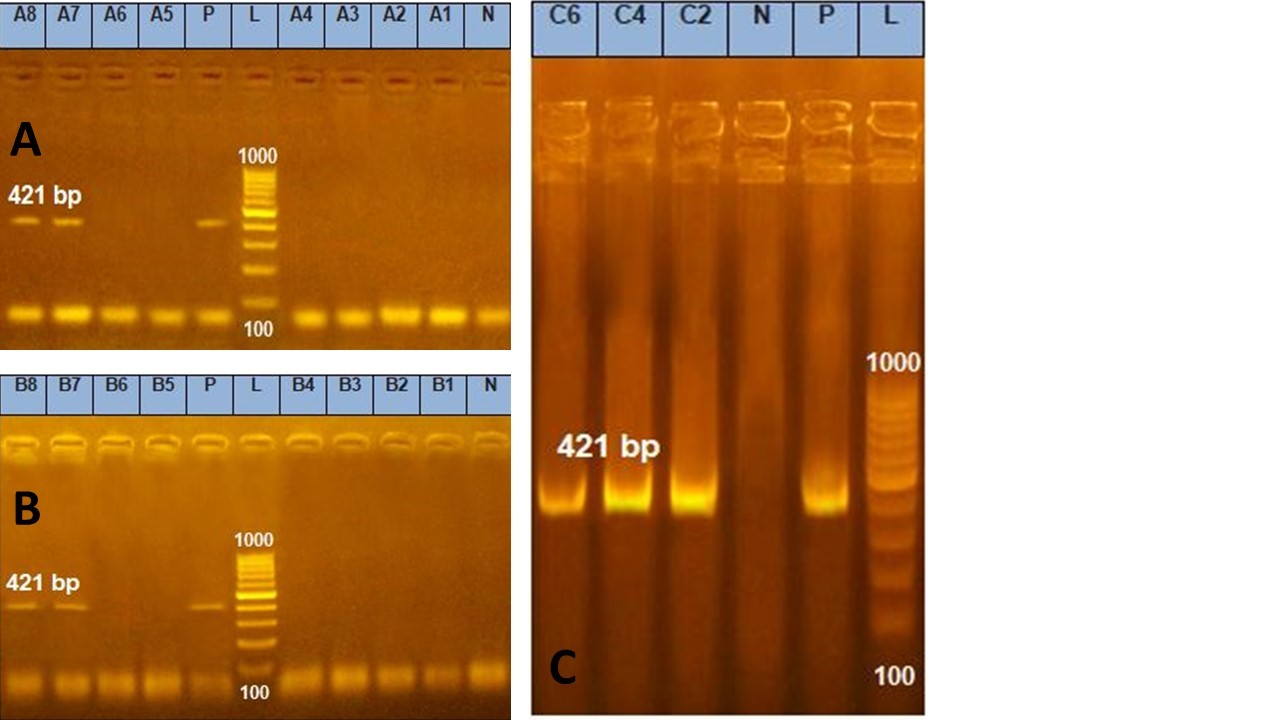
Figure 2. Agarose gel electrophoresis of PCR amplification products of cytK enterotoxin gene
-
Amplification of 421 bp products of cytK enterotoxin gene among group A. Lane L:100-1000 bp DNA molecular size marker , Lane P: Control positive Bacillus cereus cytK enterotoxin gene at 421 bp and Lanes 7 and 8: Positive Bacillus cereus isolates for cytK gene.
-
Amplification of 421bp products of cytK enterotoxin gene among group B for Bacillus cereus. Lane L:100-1000 bp DNA molecular size marker, Lane P: Control positive Bacillus cereus cytK enterotoxin gene at 421bp and Lanes 7 and 8: Positive Bacillus cereus samples for cytK gene.
-
Amplification of 421 bp products of cytK enterotoxin gene among group C. Lane L:100-1000 bp DNA molecular size marker, Lane P: Control positive Bacillus cereus cytK enterotoxin gene at 421bp and Lanes 2,4 and 6: Positive Bacillus cereus samples for cytK gene.
In recent decades, Bacillus cereus has become a significant foodborne pathogen, producing two separate illnesses by developing one emetic toxin and three other enterotoxins (14).
The results revealed that B. cereus was detected in 17 positive examined food samples [Kibda sandwiches, Sausage sandwiches, Chicken luncheon, Meat luncheon and chicken shawarma sandwiches) showed an incidence of 22.6%. These findings were lower than those mentioned by Yu et al. (15) isolated B. cereus with a percentage of 35% from the tested retail ready-to-eat food samples from different regions of China. The results were nearly similar to those of Tewari et al. (16). isolated 29 B. cereus isolates from 94 raw meat and meat product samples (30.9%), but our results were higher than Mostafa et al. (17), who observed B. cereus strains in 11.1% of meat products (chicken luncheon, beef luncheon, kofta, minced meat, beef burger and sausage).
The data revealed that from the 17 positive food samples, 40 % were positive for Bacillus cereus isolated from Kibda sandwiches, 26.6% were positive for Bacillus cereus isolated from Sausage sandwiches, 20% were positive for Bacillus cereus isolated from Chicken luncheon, 13.3% were positive for Bacillus cereus isolated from both Meat luncheon and Chicken shawarma sandwiches. These results are nearly similar to those mentioned by Mousa et al. (18) isolated Bacillus cereus with a percentage of 17.1% from luncheon samples and 25.7% from Sausage, and Samir et al. (19), recorded 20% incidences in luncheon samples, El Rahman et al. (20), reported B. cereus was 16% in shawarma sandwiches, and Mostafa et al. (17), isolated it in sausage by 23.7% & in beef luncheon by 10.5%, our findings were lower than those mentioned by Abostate et al. (21), who detailed that the frequency of Bacillus cereus in Meat luncheons and Chicken luncheons was 60% & 53.3%, respectively, and El- Sayed, (22), who isolated it from the fried liver (kibda) and Shawarma in a percentage of 88.57 and 97.14, respectively. Also, Ahmed et al. (23), found an incidence of 26.7%, and 46.7% in Luncheon and Sausage. Finally, the recorded results were higher than those reported by Meshref, (24), who mentioned that the incidence of Bacillus cereus in the examined samples of Shawarma and Kibda sandwiches was 4% for both. Researchers' varied findings may be a result of regional, seasonal, sample, and methodological variations. Additionally, the level of contamination is influenced by the processing safety measures used. Bacillus cereus is to blame for numerous outbreaks of food borne illnesses since it contains enterotoxins and emetic toxins. Two different food-borne disorders may be brought on by several toxins, according to the descriptions. The diarrheal form of the disease has been associated with hbl, nhe, and a variant of the single cytotoxin K (25). According to several studies, many bacterial infections can contaminate ready-to-eat meals through food preparation surfaces (26, 27) Antibiotics cannot enter bacteria’s cells because of proteins in their S-layer, which dramatically increases the pathogenicity of the bacterium (28).
B. cereus is responsible for a small percentage of food-borne diseases (2–5%), which result in severe nausea, vomiting, and diarrhea. When food is cooked incorrectly, the bacterial endospores survive, which leads to bacillus foodborne diseases. Some B. cereus spores may withstand cooking at temperatures lower than or equal to 100 °C (212 °F) (29).
There are two sorts of B cereus nourishment harming: the diarrheal frame, which is brought on by expending a part of bacterial cells or their spores in sullied nourishment, and the emetic form, which is brought on by consuming food contaminated with the toxin already created. Abdominal pain and diarrhea are the main symptoms of the diarrheal kind after an 8 to 16-hour incubation period (30). The toxin (cereulide) that frequently causes the emetic shape is warm steady and causes queasiness and spewing 1–5 hours after consumption (31).
B. cereus isolates were characterized using gene profiling techniques that targeted the genes Nhe, Hbl, cytK, and ces (13, 32, 33), According to PCR assays, the groEL gene for detection of Bacillus cereus and different virulence genes (nheA and cytK) of 22 different samples divided into three groups (group A represent isolates, group B represent samples taken directly from food related to isolates and group C represent samples taken directly from food of negative isolation results). Our results showed that all strains of group A & group B carried groEL gene by 100%, while group C carry the groEL gene by 50%, while the enterotoxins genes nhe & cytK were presented by 75% & 25% in group A & group B but in the group, C is represented by 50% for each. Nearly similar results were recorded by Berthold-Pluta et al. (34), who showed that the event of toxigenic B. cereus strains in all tried advertise items, of plant beginning were 70.7% for nhe and 82.7% for nhe in creature origin, and Hwang and Park, (35), who found 20 % of isolates from Infant formulas harboring cytK gene, higher results were recorded by Yu et al. (15), who tried retail ready-to-eat nourishment tests from diverse districts of China and found 83% of the disconnected strains harboring the enterotoxin-encoding nheABC quality clusters, The cytK quality was display in 68% of isolates (15). Tewari et al. (16), found toxin gene nhe by 89.7%, Rather et al., (36) detected the incidences of various enterotoxigenic genes of Bacillus cereus in meat and meat products were 96.61%, 96.61%, 93.22% and 67.78% for nheA, nheB, nheC and cytK respectively, El- Sayed, (22), recorded nhe and cytk genes in all examined samples. Also, In our research, the most predominant gene was nheA, followed by cytK in accordance with Mostafa et al. (17). In our study, we found that the detection of B. cereus directly from food by PCR methodology has resulted in a rapid and sensitive test in the detection of B. cereus and some of its enterotoxins. Here, PCR tests enable the identification of negative isolation samples, which cuts down on time-consuming manual testing. Our findings demonstrated that B. cereus is present in various foods and that the isolated strains have a variety of pathogenic genes that could potentially raise the risk of food borne illnesses.
Using the PCR strategy directly from food would allow for more efficient and rapid testing in the detection of Bacillus cereus& its enterotoxins, which should help understand the potential toxigenicity of B. cereus in food. Finally, it can be concluded that it is important to take strict hygienic measures in handling and preparation to avoid contamination with Bacillus cereus and its toxins. Also, proper temperature cooking destroys the vegetative cells of B. cereus.
'Not applicable.’
'Not applicable.’
Mayada A.M. Abou Zeid found a research idea, planned the study design, performed the bacteriological and molecular examinations, and drafted the manuscript. AbdElhafez Samir and Asmaa Ezzat Hassan shared the molecular examinations, shared designed work, and shared a collection of samples and helped the manuscript preparation. All authors approved the final manuscript.
Conflicts of Interest
Regarding this paper's research, writing, and/or publication, the authors declare that they have no potential conflicts of interest.
Received: 2023/01/20 | Accepted: 2023/04/25 | ePublished: 2023/06/26
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |