BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1864-en.html


 , Mohammad Rezaei Zadeh Rukerd1
, Mohammad Rezaei Zadeh Rukerd1 

 , Tayebe Shamsizadeh Meymandi1
, Tayebe Shamsizadeh Meymandi1 

 , Reza Sinaei2
, Reza Sinaei2 

 , Farhad Sarafzadeh3
, Farhad Sarafzadeh3 

 , Hamid Abu Saeedi3
, Hamid Abu Saeedi3 

 , Mehrdad Farokhnia3
, Mehrdad Farokhnia3 

 , Maysam Yousefi3
, Maysam Yousefi3 

 , Iman Ghasemzadeh3
, Iman Ghasemzadeh3 

 , Ali Saeedpor1
, Ali Saeedpor1 

 , Bijan Ahmadi1
, Bijan Ahmadi1 

 , Mohammad Mehdi Lashkarizadeh4
, Mohammad Mehdi Lashkarizadeh4 

 , Nafise Pishgooie1
, Nafise Pishgooie1 

 , Mohsen Nakhaie5
, Mohsen Nakhaie5 


2- Endocrinology and Metabolism Research Center, Institute of Basic and Clinical Physiology Sciences, Kerman University of Medical Sciences, Kerman, Iran
3- Department of Internal Medicine, Faculty of Medicine, Afzalipour Hospital, Kerman University of Medical School, Kerman, Iran
4- Colorectal Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
5- Physiology Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran ,
In December 2019, following the incidence of many cases of viral pneumonia of unknown origin, a deep sequencing analysis performed on lower respiratory tract samples indicated the appearance of a novel coronavirus, which was officially named 2019-nCoV as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by International Committee on Taxonomy of Virus (ICTV) (1-3). Later, the World Health Organization named this disease Coronavirus Disease 2019 (COVID-19) (4). COVID-19 started spreading from Wuhan, Hubei, China, to the whole world in December 2019 (5). Eventually, on March 11, 2020, the World Health Organization (WHO) officially declared the outbreak of this virus as a pandemic (6).
Coronaviruses are enveloped containing a non-segmented positive-sense, single-stranded RNA belonging to the coronaviridae family that can infect humans and other mammals (7). Some coronaviruses can be transmitted from animal reservoirs to humans and cause disease outbreaks (8, 9).
The incubation period of this disease varies from 1 to 14 days most of the time, and in the latest studies, the asymptomatic person becomes symptomatic after about 5 to 8 days (1, 10, 11).
Most people with COVID-19 have only mild symptoms. About 10-15% of them have more severe symptoms that require hospitalization and receive oxygen, and only 3-5% need support in the intensive care unit (ICU) (12). The main symptoms in the first patients were fever, dry cough, myalgia, fatigue, and dyspnea. Approximately 18.5% of patients in China developed more serious complications such as acute respiratory distress syndrome (ARDS), septic shock, and coagulation dysfunction (1, 7, 13).
Patients with severe COVID-19 pneumonia and the specific symptoms caused by SARS-CoV2 also show non-specific manifestations caused by the inflammatory responses. This hyperinflammatory status is caused by increased cytokines and pro-inflammatory factors (14, 15). Cytokine Release syndrome (CRS) plays a vital role in the progression of pneumonia caused by COVID-19 to acute respiratory failure and multiorgan failure, which is called a cytokine storm. Many cytokines participate in a cytokine storm, including IL-1, IL -2, IL -6, IL -10, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and GM-CSF (14, 16, 17).
IL-6 is a pro-inflammatory compound that causes inflammation in two ways: 1. cis pathway: interleukin-6 binds to its membrane receptor (mIL-6R) and gp130 and causes activation of Janus kinases (JAKs) signal transducer, transcription 3 (STAT3), and ultimately the activation of the cellular and innate immune system, which plays a role in cytokine release syndrome. 2. Trans pathway: Interleukin-6 binds to its soluble receptor (sIL-6R) and gp130 in endothelial cells, which causes the development of cytokine storm through the activation of vascular endothelial growth factor (VEGF), monocyte chemoattractant protein-1 (MCP- 1). The release of VEGF and the increase in vascular permeability due to the decrease in E-cadherin causes an increase in vascular permeability and, ultimately, acute respiratory failure syndrome (18-20).
Tocilizumab is a monoclonal antibody against the IL-6 receptor, inhibiting IL-6 signaling after connecting with its receptor. Tocilizumab is used to treat rheumatoid arthritis, juvenile inflammatory arthritis, and systemic inflammatory responses caused by the release of a massive amount of pro-inflammatory cytokines (21-23).
Various studies worldwide have been recorded regarding evaluating the effectiveness of Tocilizumab and its different effects on various prognostic factors, producing different results. In this study, we intend to investigate the effects of Tocilizumab on several aspects, such as the period of hospitalization, the need for mechanical ventilation, the patient's condition at the time of discharge, and the blood level of various factors for the first time in southeast Iran.
Study Population
In this case-control study, 300 patients were included by simple random sampling method in the form of 2 groups; the control group included 150 patients, and the case group contained 150 patients. Inclusion criteria consisted of patients referred to the infectious diseases department of the Afzalipour Hospital (Kerman, Iran) with a positive reverse transcriptase polymerase chain reaction (RT-PCR) for COVID-19 and evidence of severe pneumonia from November 2021 to January 2022. The exclusion criteria for this study contain 1. Any active bacterial or fungal infection 2. Positive blood and urine cultures 3. History of chronic obstructive pulmonary diseases 4. History of active tuberculosis 5. Active skin ulcer 6. Severely ill (shock, encephalopathy, myocardial damage, coagulation, and kidney disorders) 7. Abnormalities in liver tests in the form of liver enzymes above two times normal 8. Glomerular Filtration Rate (GFR) less than or equal to 30 9. Lack of consent to conduct the study.
Study Design
In this study, 300 patients were included by simple random sampling method in the form of 2 groups; the case and control group. The Control group was treated with remdesivir, dexamethasone, heparin, and supportive therapy, while the case group was treated with intravenous (IV) Tocilizumab in addition to those treatments. The treatment protocol we used was determined by a weight-based dose strategy (8 mg/kg, maximum dose 800 mg), followed by a second infusion in the patients who had a respiratory worsening after 24 hours. At that time, the patients of both groups are compared in terms of the patient’s discharge condition, the need for mechanical ventilation and oxygen, the hospitalization period, and the serum level of White blood cell count (WBC), lymphocyte, Ferritin, Lactate dehydrogenase (LDH), C-reactive protein (CRP), and D-dimer.
Statistical Analysis
The minimal sample size of 300 patients was calculated, assuming a type I and II error of 5 and 20%, respectively. Study data were analyzed using SPSS 26 software (IBM, Armonk, NY, U.S.). Qualitative variables were reported as numbers (percentages). In contrast, quantitative variables were noted as mean (standard deviation) and median (range between quarters) values if there was a normal or abnormal distribution, respectively. All mean comparison tests were performed two-way. Chi-Square and Mann-Whitney tests were used in this analysis. For all analyses, p<0.05 indicates a statistical significance difference.
This study included 150 patients in the case group (under treatment with Tocilizumab) and 150 in the control group (standard treatment group). In the case group, 150 patients with an average age of 62 ± 7.6 years were included in the study, of which 87 were men (58%) and 63 were women (42%). In the control group, 150 patients with an average age of 59 9.1 years were included in the study, of which 92 were men (61.3%) and 58 were women (38.7%).
In comparing the patient’s discharge condition, in the case group, 117 patients (78.0%) were discharged with full or partial recovery, and 33 patients (22.0%) also died. In the control group, at the time of discharge, 129 patients (86%) had a full or partial recovery, and 21 patients (14.0%) died (P=0.169) (Table 1).
Table 1. Compare the patient’s discharge condition of the case and control group
| Discharge status | P-value | ||||
| Full recovery | Partial recovery | Dead | |||
| Groups | case | 90(60.0%) | 27(18.0%) | 33(22.0%) | 0.169 |
| control | 103(68.7%) | 26(17.3%) | 21(14.0%) | ||
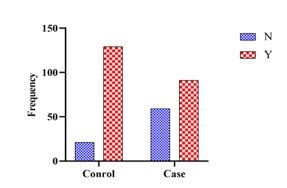
In the comparison of the need for mechanical ventilation in both case and control groups, 91 patients in the case (60.7%) and 129 patients (86.0%) in the control group required mechanical ventilation during hospitalization (P<0.0001) (Figure 1).
Contrary to the evaluation of the length of hospital stay in the case and control groups, the mean number of hospitalization periods in the case group was 6.21±4.08 days, while in the control group was 8.41±4.23 days (P<0.0001) (Table 2).
Figure 1. The prevalence of required mechanical ventilation in the case and control groups (Y: YES ; N: No)
Table 2. Compare the hospitalization periods in the case and control groups
| P-Value | Median | Mean | N | Group |
| <0.0001 | 4.00 | 6.21 | 150 | Case |
| 10.00 | 8.41 | 150 | Control |
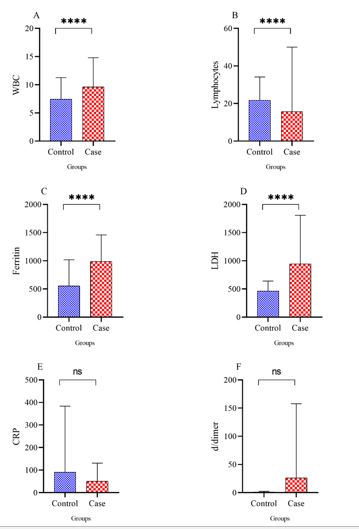
In comparing the serum level of WBC, lymphocyte, ferritin, LDH, CRP, and D-dimer in the case and control groups, the average serum level of WBC was 9.61±5.17 cells/μL in the case group and 7.44±3.81cells/μL in the control group (P<0.0001). The average percentage of lymphocytes was 15.65±15.65 in the case group and 21.18±11.95 in the control group (P<0.0001). The mean ferritin serum level was 986.85±463.29 µg/L in the case group and 554.55±463.29 µg/L in the control group (P<0.0001). The mean serum level of LDH in the case group was 942.13±859.78 units/L; in the control group, it was 462±179.21units/L (P<0.0001). The mean serum level of CRP in the blood of case group patients was 50.63±18.4 mg/dL, while this number was 80.79±19.4 mg/dL in the control group (P=0.278). The mean serum level of D-dimer in case group patients was 25.31±18.4 ng/mL, while this number was 26.1±19.6 ng/mL in the control group (P=0.191) (Figure 2).
Figure 2. Compare WBC, lymphocyte, ferritin, LDH, CRP, and D-dimer levels in case and control groups
IL-6 plays a very important pivotal role in the pathogenesis of the inflammatory system in patients with severe pneumonia caused by COVID-19. Its block has been evaluated in several studies in the form of randomized controlled trials (RCT) and uncontrolled clinical trials (14, 24-26). This study describes the effect of IV Tocilizumab as a monoclonal antibody against the IL-6 receptor on clinical outcomes of COVID-19 patients in Iranian tertiary hospitals compared to patients receiving standard management. No safety concerns associated with Tocilizumab in this population were studied.
In this study, 300 people were studied in case and control groups. The chi-square statistical test showed no significant difference between the two groups in terms of age and gender (P=0.644 & P=0.624, respectively). Similar to our study, in the studies of Campochiaro et al., Guaraldi et al., and Rosas et al., there was no statistically significant difference between the case and control groups regarding age and gender (23, 26, 27).
There was no significant difference in the patient's discharge conditions (death, partial recovery, full recovery) in the case and control groups (P-Value=0.169). Nevertheless, a larger part of the patients in both groups experienced clinical improvement and was finally discharged from the hospital. Although there is a significant difference in the need for mechanical ventilation in the case and control groups, it was less in the Tocilizumab treatment group (P<0.0001). In agreement with our study, in Corrado Campochiaro et al.'s study, higher baseline PaO2:FiO2 was predictive of clinical improvement on day 28, and it was concluded that on day 28, clinical improvement and mortality were not statistically different between Tocilizumab and standard treatment patients (26). In the results of the RECOVERY trial conducted on 21,550 patients who did not need mechanical ventilation at the beginning of hospitalization, the patients treated with Tocilizumab had less mortality. They were less likely to reach the endpoint of invasive mechanical ventilation (28). According to the study by Carlos Salama et al., treatment with Tocilizumab reduces the need for mechanical ventilation and death in hospitalized patients who are not treated with mechanical ventilation. However, it does not affect increasing survival (29). Rosas et al.'s study also showed that the use of Tocilizumab does not affect the mortality rate in the first 28 days after the disease (27). In agreement with our study, in the study of Guaraldi et al., treatment with Tocilizumab in hospitalized patients was associated with a reduction in the use of invasive mechanical ventilation (23).
In the current study, there was a significant difference between the average length of hospital stay in the case and control groups, which means that the people treated with Tocilizumab had fewer days of inpatient hospital care and were discharged from the hospital earlier (P-Value<0.0001). Similar to our study, in Minihan et al.'s and Zhang et al.'s study, patients treated with Tocilizumab had a shorter length of stay in the intensive care unit (ICU) and hospital (28, 29). Contrary to the results of our study, in Rosas et al. article, Tocilizumab and remdesivir treatment did not change the length of hospital stay compared to placebo and remdesivir (30). In the study of Carlos K H Wong et al., Tocilizumab does not affect the patient's clinical condition and discharge time, which is against our study (31).
In the present study, the number of WBCs, serum ferritin, and LDH at the time of admission were significantly higher, and the number of lymphocytes was significantly lower in the group treated with Tocilizumab (P-Value < 0.0001). D-dimer serum level and CRP serum level were not significantly different between the two groups (P-Value= 0.278 and P-Value= 0.191). Consistent with Klopfenstein et al., patients treated with Tocilizumab, despite poor biological symptoms such as severe lymphopenia, had lower ICU admissions and a need for mechanical ventilation (32).
We considering that this research was conducted for the first time in the southeast of Iran. So, it had restrictions such as low sample size and, and the lack of similar research in the region in order to more accurately compare the results of this research with other regional researches. Increasing the studied sample size indicates more meaningful and pointless relationships that can be presented about the intravenous Tocilizumab treatment in COVID-19. It is suggested that future supplementary researches should be carried out in a larger sample size, in different geographical areas, and a more extended period.
In our study, there was no significant difference in the patient's discharge conditions (death, partial recovery, full recovery) in the case and control groups (P=0.169).
The difference between the average length of hospital stay in the case group was significantly lower (P<0.0001).
The number of WBCs, serum ferritin, and LDH at the time of admission were significantly higher, and the number of lymphocytes was significantly lower in the group treated with Tocilizumab (P<0.0001). So, as a result, although there was a significant difference between the average length of hospital stay and required invasive mechanical ventilation in the case and control groups despite the presence of poor biological symptoms such as severe lymphopenia in admission, there was no significant difference in the patient's discharge conditions and mortality rate.
We want to thank all the patients involved in this study and devote this work to the retention of health care workers who sacrificed day and night to save the lives of patients with COVID-19.
The study protocol was approved by the Research Ethics Committee of Kerman Medical University (IR.KMU.AH.REC.1400.129), and written informed consent was obtained from each participant.
SSh – concept and study design, supervision on data collection, validation of data source and contents, interpretation of data. MRZR – statistical analysis, writing statistical components, interpretation of data, writing the original draft, revising the manuscript. TShM – acquisition, data collection and tabulation. RSi and FSa and HASa and MFa and MYo and IGa – supervision on data collection, validation of data source and contents, interpretation of data, formal analysis. ASa and Bah and MMLa and NPi– acquisition, data collection, tabulation, formal analysis, review the final draft. MNa – methodology, supervision, formal analysis, review and editing final draft. The author(s) read and approved the final manuscript.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2022/08/28 | Accepted: 2022/12/21 | ePublished: 2023/03/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |