BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1777-en.html


 , Zainab Sabah AL-Khalidi1
, Zainab Sabah AL-Khalidi1 

 , Estabraq Hassan Almuhanna1
, Estabraq Hassan Almuhanna1 

 , Hiba Ridha Hussein1
, Hiba Ridha Hussein1 

 , Abbas F. Almulla2
, Abbas F. Almulla2 

 , Haider Ali Alnaji1
, Haider Ali Alnaji1 


2- Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand ,
Otitis media (OM), often known as middle ear inflammation, is a group of inflammatory and bacterial infections that influence the middle ear (1, 2). The burden of the illness is significant in resource-restricted situations, while human and physical diagnostic and treatment resources remain inadequate (2, 3). Various infectious micro-organisms can cause OM, such as bacteria, viruses, and fungi (4). Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus are the most common pathogens in chronic otitis media with effusion (COME), which form biofilms with other otopathogens (4, 5). This biofilm may trigger innate inflammatory responses and contributes to the chronicity of OM and its progression to COME despite the proper treatment (6, 7).
Pseudomonas aeruginosa is one of the opportunistic bacterium agents which is implicated in the pathogenesis of OM; these bacteria own several types of virulence factors (8). In this regard, Mansoor et al. found that most of the isolates in the COME are P. aeruginosa (9), and it has an inherent resistance to a broad spectrum of drugs (9, 10). Enzyme synthesis, efflux pump overexpression, porin deficiencies, and target-site changes have all been reported as resistance mechanisms in P. aeruginosa (10). Moreover, increased expression of AmpC β-lactamase is frequently linked to β-lactamase resistance in P. aeruginosa (11, 12). In addition, cross-resistance may play a role in P. aeruginosa's multidrug resistance (12).
Indeed, P. aeruginosa is intrinsically resistant to several antimicrobials and can acquire mobile genetic elements (MGEs) due to the chromosomally encoded resistance genes (13, 14). This last mechanism is crucial in forming and disseminating drug resistance (10). Bacteria acquire genes to survive in unfavorable environments, namely when antibiotics are present (10). Plasmids and other MGEs are essential in this process since they serve as vectors for capturing and disseminating resistance genes. Exotoxin A, exoenzyme S, elastase, and sialidase are some virulence factors identified in P. aeruginosa. They are tightly controlled by cell-to-cell signaling networks (15).
Exotoxin A is encoded by the toxA gene and inhibits protein biosynthesis by transferring an ADP-ribosyl moiety to elongation factor 2 of eukaryotic cells (16). Moreover, neuraminidases, also known as sialidases, are encoded by the nan1 gene, which is the main enzyme that hydrolyzes the linkage of terminal sialic acids on various sialoglycoconjugates to generate free sialic acid (16). It represents the terminal sugar residue of many glycan chains on host cell surfaces involved in cell-cell recognition; thus, this enzyme has an essential role in the implantation of the bacterium (17). However, no studies have investigated the existence of nan1 and tox A genes in the P. aeruginosa isolated from OM.
Hence, in the current study, we aim to detect the nan1 and tox A genes in P. aeruginosa isolates from chronic otitis media (COM) patients. The hypothesis is that these two genes are present in P. aeruginosa isolated from OM patients and they are part of the resistance system to antibacterial treatments.
Patients and Clinical Specimens' Collection
In this study, we have recruited 130 patients with COM whose range of age is (1- 85 years) from both genders and over a specific time (September 2020 to January 2021). Patients were recruited from Al-Sadder Medical City and Al- Hakeem General Hospital in Najaf city, Iraq. A well-trained otorhinolaryngologist performed the patients' diagnosis based on the ICD-10 criteria. Ear swabs were collected from all patients with a swap collection tube from JSHXRT company, China. All samples were further processed according to the standard criteria reported by Cheesbrough (18). We followed the Iraqi and international ethics and privacy laws to perform the current study. Before participating in the present study, written informed consent is obtained from each individual or their parents. In the current study, the College of Medicine at the University of Kufa in Iraq has provided the IRB approval (617/2020) compliant with the Declaration of Helsinki's International Guideline for Human Research Protection.
Isolation and Identification of the Bacterial Isolates
After the samples were collected, we transferred them to the laboratory to inoculate on appropriate media, such as MacConkey's agar and blood agar plates, and incubated them at 37°C for 18-24 hours. Gram staining, colony morphology on different media, and biochemical tests were used to make the primary diagnosis of isolates. One hundred fifteen isolates were identified and validated using the Vitek-2 system, which uses a kit (GP-ID cards) for Gram-positive bacteria and 15 isolates as Gram-negative bacteria identified by (GN-ID cards) with ID message confidence levels ranging from very good to outstanding (probability percentage from 95 to 99). In addition, using the VITEK2-automated system, the final identification of bacteria isolates was confirmed biochemically. We examined the antibacterial resistance of P. aeruginosa isolates by VITEK 2 automated system (Table 1). Susceptibility profiles were performed with 12 antibiotics in the specific AST cards, including Amoxicillin-clavulanic acid Ceftriaxone, Cefotaxime, Cefepime, Ceftazidime, Azithromycin, Levofloxacin, Gentamycin, Amikacin, Tobramycin, Ciprofloxacin, and Imipenem.
DNA Extraction
The sample preparation of DNA extraction was done as follows: a) one ml of the overnight bacterial growth on brain heart infusion (BHI) broth was taken and placed in a 1.5 ml microcentrifuge tube and centrifuged at 10000 rpm for 1 minute, b) the supernatant was thrown away, and the bacterial cell pellets were utilized to extract genomic DNA, c) genomic DNA was extracted according to the manufacturer's procedure using a commercial extraction kit (Genomic DNA Promega Kit). UV spectroscopy was used to assess the quality and quantity of DNA isolated from bacterial cultures. The Optical Density (OD) at 260/280 nm was used to evaluate DNA purity on a Shimadzu UV-1800 Double Beam UV/Visible Scanning Spectrophotometer.
PCR Amplification
As previously described (20), DNA samples (5 μL) were subjected to PCR apparatus in a 25μL reaction mixture using SureCycler 8800 (Agilent Technologies, Inc. USA). The following specific thermal cycling pattern was used to amplify the nan1 gene: 35 cycles consisting of 5 min initial denaturation, 30 seconds (s) at 94°C for denaturation, 60 S at 58°C for annealing, 2 min at 72°C for extension, and 5 min for the final extension. tox A, gene amplification was performed following the following conditions: initial denaturation step at 94°C for 5 min; 30 cycles of denaturation at 94°C for the 30 s, annealing at 55°C for 60 s, extension at 72°C for 1 min, followed by a final extension step at 72°C for 5min. Table 2 lists the specific sequence primers used by Alpha DNA Company (Canada). Electrophoresis was used to analyze DNA fragments in a 1% agarose gel at 70 V for 1.5 h. in TBE 1X containing 4 µL of 10 mg/mL ethidium bromide using a 100-bp DNA ladder (Bioneer, Korea) as a size marker. On an ultraviolet light transilluminator (Cleaver, UK), a single band was seen at the appropriate spot; bands were photographed using a gel documentation system (Cleaver, UK).
Table 1. Percent of resistance of P. aeruginosa isolates against tested antibiotics
| Percent of total isolates | Type of Antibiotic | No. |
| 93.75 | Amoxicllin-clavulinc acid | 1 |
| 81.25 | Ceftriaxone | 2 |
| 70.83 | Cefotaxime | 3 |
| 52.08 | Cefepime | 4 |
| 52.08 | Ceftazidime | 5 |
| 52.08 | Azithromycin | 6 |
| 18.75 | Levofloxacin | 7 |
| 18.75 | Gentamycin | 8 |
| 18.75 | Amikacin | 9 |
| 29.16 | Tobramycin | 10 |
| 16.66 | Ciprofloxacin | 11 |
| 8.33 | Imipenem | 12 |
Table 2. Primers used in this study
| Gene | Primer sequence (5'-3') | Amplicon size (bp) | Reference |
| nan1 | F: AGGATGAATACTTATTTTGAT R: TCACTAAATCCATCTCTGACCCGATA |
1316 |
(20) |
| ToxA | F: GGTAACCAGCTCAGCCACAT 3’ R: TGATGTCCAGGTCATGCTTC 3 |
352 |
(20) |
Distribution and Characteristics of Patients
Table 3 shows the patients' distribution and demographic characteristics across two hospitals that provided the samples. Most of the patients were recruited from AL-Sadar Medical City in Najaf province. We found that 74 (57%) specimens were acquired from males and 56 (43%) from females. Based on the residency, 79.6% of specimens were taken from urban patients, whereas 51.9% of from rural. Table 4 shows the patient distribution by age and gender. We found the OM most prevalent among adults aged 21-30 and the least prevalent among older adults 51-80 from both genders. Table 5 revealed that most bacterial isolates were gram-positive 115, taken from urban people, while only 15 isolates showed a Gram-negative test.
Table 3. Distribution and characteristics of patients recruited in the presented study
| AL-Sadar Medical City | AL-Hakeim Teaching Hospital | Variables | |||
| 80 | 50 | No. | |||
| 46/34 | 28/22 | Gender (M/F) | |||
| 32/48 | 19/31 | Residency (R/U) | |||
| 70/10 | 45/5 | Diagnosis (GP/GM) | |||
No.: Number, M: Male, F: Female, R: Rural, U: Urban, GP: Gram-Positive, GM:
Gram Negative.
Table 4. Age-Gender distribution of the patients.
| Age(year) | Male | Female | Total % |
| 1-10 | 13 | 11 | 24(18.4%) |
| 11-20 | 12 | 8 | 20(15.3% ) |
| 21-30 | 22 | 18 | 40 (30.7 %) |
| 31-40 | 10 | 7 | 17(13% ) |
| 41-50 | 6 | 10 | 16 (12.3% ) |
| 51-80 | 11 | 2 | 13( 10% ) |
| Total | 74 (57%) | 56 (43%) | 130 (100%) |
Table 5. Distribution of type of bacteria with the residence of patients
| Residence | Positive | Negative | Total % |
| Urban | 71(54.6%) | 8(6.1%) | 79(60.7%) |
| Rural | 44(33.8%) | 7(5.4%) | 51(39.2%) |
| Total | 115(88.4%) | 15(11.5%) | 130(100%) |
Detection of the nan1 Gene
The nan1 gene (1316 bp) of P. aeruginosa was detected in the isolates using the PCR technique; the findings indicate that 19 (38.77%) of the isolates tested positive for the gene, as shown in Figure 1.
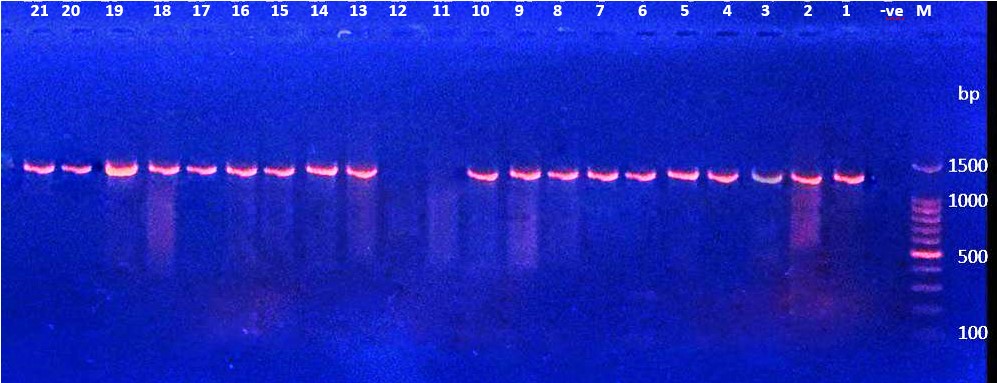
Figure 1. Ethidium bromide-stained agarose gel electrophoresis of PCR products from extracted total DNA of P. aeruginosa using primer nan1 gene with product 1316bp. The electrophoresis was performed at 70 volts for 1.5-2hr. lane (M), DNA molecular size marker (1500 bp ladder). Lane (-ve) shows negative controls. Lanes (1 to 2 except 11, 12) show positive results with gene nan1.
Detection of toxA Gene
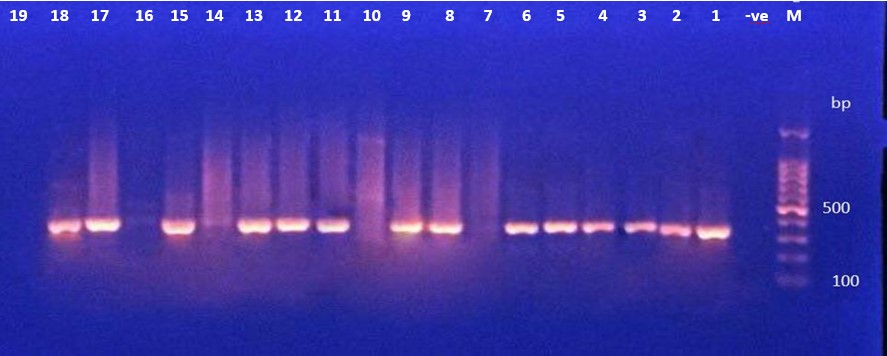
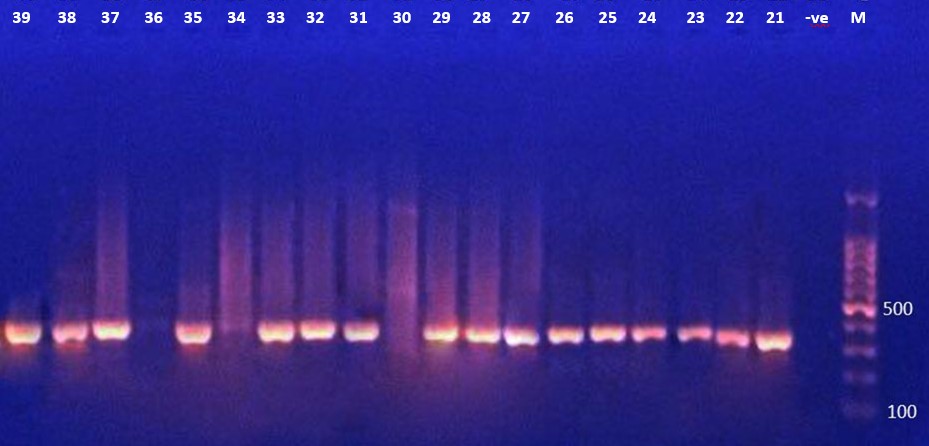
The presence of the toxA gene (352 Bp) in P. aeruginosa isolates was determined by PCR amplification. The data in Figure 2A, 2B, 2C showed that 33 (67.3%) of the bacterial isolates tested positive for the toxA gene.
|
Figure 2 (A). Ethidium bromide-stained agarose gel electrophoresis of PCR products from extracted total DNA of P. aeruginosa using primer toxA gene with product 352bp. The electrophoresis was performed at 70 volt for 1.5-2hr. lane (L), DNA molecular size marker (1500 bp ladder). ). Lane (-ve) show negative controls. Lanes (1 to 18 except 7,10,14,16 and 19) show positive results with gene toxA gene. |
|
|
Figure 2 (B). Ethidium bromide-stained agarose gel electrophoresis of PCR products from extracted total DNA of P. aeruginosa using primer toxA gene with product 352bp. The electrophoresis was performed at 70 volt for 1.5-2hr. lane (L), DNA molecular size marker (1500 bp ladder). ). Lane (-ve) show negative controls without DNA. Lanes (21 to 39 except 30 and 36) show positive results with gene toxA gene. |
|
|
Figure 2 (C). Ethidium bromide-stained agarose gel electrophoresis of PCR products from extracted total DNA of P. aeruginosa using primer toxA gene with product 352bp. The electrophoresis was performed at 70 volt for 1.5-2hr. lane (L), DNA molecular size marker (1500 bp ladder). ). Lane (-ve) show negative control witout DNA. Lanes 40,45 and 46 show positive results with gene toxA gene. Lanes41,42,43,44,47 and 48 were negative |
The major finding in the presented study was that most isolates of P. aeruginosa from OM patients showed the presence of nan1 and toxA genes. We found that 19 (38.77%) isolates carried the nan1 gene, as shown in Figure 1. Khattab et al. also found this gene in four out of ten (40%) pulmonary tract isolates and likewise (40%) burn isolates, but not in blood infection isolates (19). These results confirmed the presence of this gene in P. aeruginosa, which is considered one of the virulence factors (19). P. aeruginosa has been reported as the most prevalent cause of OM, followed by Staphylococcus aureus, and infection rates for the first two types are the greatest among all instances of infection (20). However, other studies have found that S. aureus is the most common cause of OM (21, 22). The pattern of microbiological distribution differs in OM due to geographical variance, local antibiogram pattern, and differences in climate, community, and patient characteristics (23).
All protein virulence factors in the P. aeruginosa PAO1 strain are chromosomally encoded, and their gene sequences and locations are well known (earlier called "P. aeruginosa strain 1") in the genome's sequence (24). The coexistence of antimicrobial resistance determinants, which virulence factors may partially explain in P. aeruginosa isolates, is a serious issue in treating this bacterial infection (25). It is essential to detect all mediators in multidrug-resistant organisms to determine the optimal treatment options for such infections (26).
The pathogenicity of P. aeruginosa was measured using a variety of cell-associated chemicals, including (e.g., flagella, pili, alginate capsule, and lipopolysaccharide) (27). P. aeruginosa, on the other hand, can secrete substantial amounts of extracellular components (mainly toxins like exotoxin A and exoenzymes) as well as enzymes that may contribute to pathogenicity (e.g., proteases, elastases, phospholipase C, and neuraminidases) (28). The nan1 gene encodes a critical cell-surviving protein called sialidase or neuraminidase enzyme (29). This enzyme is essential for adhesion to the respiratory tract (29). Extracellular neuraminidase aids bacterial implantation, albeit the genetic basis of this action is unknown (29). Some studies found that the nan1 gene was found in bacterial isolates from the ear and burnt swabs than in blood isolates (20, 30, 31). Differences in virulence factor gene distributions in populations increase the likelihood that some P. aeruginosa strains are more adapted to the unique conditions encountered in specific infectious locations. Hence, virulence gene expression varies according to the infection site and severity (14, 32).
Another virulence factor recruited by P. aeruginosa is endotoxin A, which is considered the pathogenic bacterium's most toxic virulence factor (33). This protein is encoded by the toxA gene of P. aeruginosa (Figure 2), and many isolates exhibit the presence of this gene. Like other extracellular virulence agents, including diphtheria, cholera, and pertussis toxin, Exotoxin A inhibits protein synthesis (34). An ADP-ribosyl transferase coats the host elongation factor-2, halting protein synthesis and resulting in cell death and hearing loss (34). Exotoxin can infiltrate the inner ear within hours, enhancing round window membrane (RWM) permeability during acute inflammation. But it decreases during persistent infections or the post-infectious phase (34, 35), which may lead to hearing loss with the onset of deterioration at 24-48 h after instillation, indicating rapid incursion (34). Therefore, treating the infection at the beginning of the illness protects against hearing damage.
The regulation of toxA expression is a complicated process involving numerous gene products. RegA, Vfr, Fur, PvdS, PtxR, and PtxS are transcriptional regulators suggested playing a role in toxA production, among other proteins (36). PtxR and PtxS are the most perplexing regulators (37). There has been no direct interaction between these proteins and the toxA promoter, and the molecular mechanism by which PtxS regulates toxA expression is unknown (36). P. aeruginosa is antibiotic-resistant due to various mechanisms, including a low-permeability membrane, efflux pumps, and a variety of beta-lactamases (38). There is also a possible dependency of antibiotic resistance to the existence of toxA and nan1 gene (37). A higher frequency of these genes in isolates resistant to treatment indicates the involvement of these genes in drug resistance (38).
In particular, P. aeruginosa was implicated in the progressive destruction of middle ear tissues through its toxins and enzymes (39). P. aeruginosa is a gram-negative bacterium with high adaptability and intrinsic resistance to antibiotics, enabling it to colonize a variety of settings (40). It has been proposed that during OM, the ear environment became suitable for the colonization of P. aeruginosa, and that difficult to eradicate or remove its toxins and enzymes, which damage the ear tissue. P. aeruginosa can avoid the host immune response by minimizing blood flow and forming a shell that protects itself (41). It has several exotoxins and enzymes that are secreted passively or through the secretion system; most importantly, exotoxin A (toxA), protease (lasA), and elastase (lasB), all these virulence factors secreted via secretion system type II (42). In Najaf city of Iraq, we found a high prevalence of OM patients due to infection with P. aeruginosa, and that probably resulting from a) increased antibiotic resistance as the people can get any antibiotic over their counter, b) lack of hygiene, especially in government hospitals that enhance the prevalence of many opportunistic bacteria such as P. aeruginosa c) the potentials of bacteria to adapt with various environments.
There are some limitations in the current study that should be considered when interpreting the present findings. First, we could not examine the association of nan1 and toxA genes with different antibiotic resistance. However, many of the isolates were resistant to some antibiotics. Further studies should examine only resistant bacteria. Moreover, real-time polymerase chain reaction (RT-PCR) should recruit to determine whether these genes are translated and produce proteins.
Our findings reveal that the bacterial isolates of P. aeruginosa from OM patients have the nan1 and tox A genes, with a high incidence of tox A among nan1. Probably these genes partly explain the resistance of the bacteria to the treatment. Furthermore, P. aeruginosa is the most cause of OM in Iraqi patients.
None.
None.
Conflicts of Interest
The authors declared no competing interests.
The isolates have been gathered from two hospitals by Maryam R. Mansor and Zainab Sabah AL-Khalidi. Estabraq Hassan Almuhanna has tested the isolates for antibiotic resistance and for identifying each species of bacteria. Haider Ali Alnaji, Maryam R. Masor, and Hiba Ridha Hussein have managed to do the PCR and Abbas F. Almulla has written Gel Electrophoresis and the article with contributions of Maryam R. Finally, Mansor and Haider Ali Alnaji. Abbas F. Almulla, Haider Ali Alnaji, and Maryam R. Mansor have updated the article.
Ethical Approval
The College of Medicine at the University of Kufa in Iraq provided the IRB approval (617/2020) compliant with the Declaration of Helsinki's International Guideline for Human Research Protection.
Availability of Data and Materials
All the relevant data is present within the current version of the study; however, upon request, any extra information will be provided by the corresponding author (AA).
Ethics Approval and Consent to Participate
We followed the Iraqi and international ethics and privacy laws to perform the current study. Before participating in the present study, written informed consent was obtained from each individual or their parents.
Received: 2022/06/7 | Accepted: 2022/10/8 | ePublished: 2023/01/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |