BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1724-en.html
2- Department of Pathobiology, Faculty of Veterinary Medicine, Lorestan University, Khorram Abad, Iran ,
Brucellosis is a zoonotic disease with a bacterial agent that is important from both health and economic aspects. Transmission of brucellosis to humans is through consumption of raw milk and contaminated dairy products or direct contact with fluids or tissues of infected animals (1). Despite control and prevention programs such as vaccination and slaughter of infected animals, Iran is one of the pandemic with high prevalence of this disease (2). Lorestan province, with about 6.5 million livestock units, accommodates 5.5% of the country's livestock population and has played an important role in the production of livestock products, which is now one of the centers with a high prevalence of Brucellosis in the country (3). The eradication of this disease depends not only on the prevention of new cases of the disease but also on the timely diagnosis of the disease in humans and animals.
Serological and bacterial culture methods, which are the most common tests for diagnosing the Brucella infection, have major drawbacks. Disadvantages of the bacterial culture method include time consuming, facilitates the risk of acquiring laboratory infection and the need for biosafety Level 3 (BSL-3) laboratories. In addition, serological methods are less specific and may provide false results. Also, the clinical similarity of the symptoms of brucellosis with many other infectious and non-infectious diseases, has led to the use of molecular methods with high sensitivity and accuracy to diagnose the strain of Brucella (2, 4). Therefore, due to the importance of cow's milk as the main source of dairy production and the important role of milk in the possible transmission of brucellosis to humans, the present study were addressed to evaluate the prevalence of Brucella spp. and B. abortus in cow milk obtained from various regions of Lorestan province using the PCR method.
Sample collection
In this study, a total of 100 raw milk samples obtained from industrial and traditional farms in 6 regions of north (Noor Abad), south (Pole Dokhtar), west (Aligoudarz, Azna and Doroud), east (Kouhdasht), northwest (Boroujerd and Alashtar) and center (Khorram Abad) of Lorestan province (Figure. 1) were randomly collected from February to June 2020. 50 ml of milk was collected from each cow after disinfecting the nipples with 70% alcohol in sterile. Then the age of the animals was recorded and finally, the samples under aseptic conditions were transferred to the laboratory of veterinary laboratory, Lorestan University on the icebox.
Figure 1. Geographical map of Lorestan province.
DNA extraction
Genomic DNA Extraction was done using 10 ml of each milk sample. Samples were centrifuged at 6000 rpm for 15 minutes. Then, 200μl of the fatty top layer was transferred to the 1.5 ml tube of each sample and using a DNA extraction kit (Gene All South Korea), the DNA extraction process was performed according to the manufacturer's protocol. The quality and concentration of the extracted DNA were evaluated by spectrophotometry with a wavelength of 260 to 280 nm, and the extracted DNA was stored in a -20 freezer for further study.
Identification of Brucella spp. and B. abortus by PCR:
To identify Brucella spp. and B. abortus, specific primers bcsp31 (31 kDa outer membrane protein) and IS711 prepared by Takapo Zist Tehran (Iran) were used, respectively (Table 1). PCR amplification was performed using PCR master kit (Ampliqon Taq DNA Polymerase Master Mix RED 1.25 mL, Ampliqon Denmark) with 25 μL mixtures containing 12.5 μL of 2X master mix, 0.5 μL of each primer, and 5 μL of the extracted DNA. For positive control represented by genomic DNA isolated from vaccine strain RB51 (Razi Vaccine and Serum Institute - Iran) and for the negative control, sterile water was added instead of nucleic acids. Further, the amplification was conducted by Bio-Rad thermocycler (Model T- 100, USA) under the following conditions: A for Brucella spp. and B for B. abortus.
Table 1. PCR primers used for Brucella spp. and B. abortus detection.
| Target gene | Primer sequence | Amplified Product size | References |
| bcsp31 |
(F) 5'-TGG CTC GGT TGC CAATAT CAA-3' | 223 (bp) | (5) |
| (R) 5'- CGC GCT TGC CTT TCA GGT CTG-3' | |||
| IS711 | (F) 5'-GACGAACGGAATTTTTCCAATCCC-3' | 498 (bp) | (6) |
| (R) 5'-TGCCGATCACTTAAGGGCCTTCAT-3' |
B: The initial step of 95°C for 5 min, followed by 35 cycles of 95°C for 75 Second, 55.5°C for 1 min, 72°C for 2 min, and finally, 72°C for 10 min.
The PCR products were separated in a 1.5% (w/v) agarose gel (Merck, Germany) containing 2.5 μg/mL gel stain (Sina Gene, Iran). Electrophoresis was performed in 0.5x Tris/Borate/EDTA (TBE) buffer for one hour at 100 V. The resulting PCR products were visualized under a UV transilluminator (E-Box, Iran) and the 100 bp DNA ladder (Smobio, Taiwan) plus was used as the molecular size marker.
Statistical analysis
Data analysis was performed using Chi-square Tests. For this purpose, SPSS software version 22 (IBM, USA) was used to perform this statistical test and the difference was considered significant with p <0.05.
100 milk samples were investigated using PCR method. Based on the results 26 (26%) milk samples were positive for Brucella spp. and 19(73%) cases of them were positive for B. abortus, respectively (Figure 2).
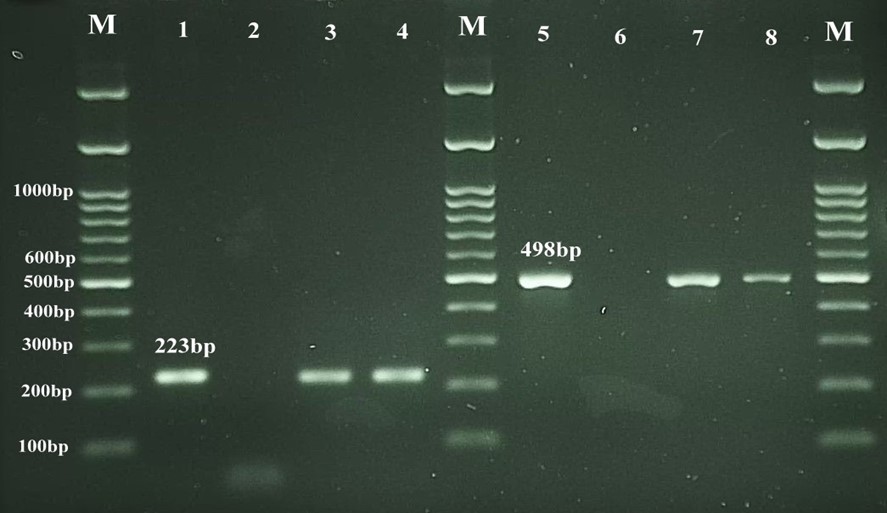
Figure 2. PCR assay for the detection of Brucella .spp and B. abortus in raw milk samples. Lane M: Standard DNA marker (100bp DNA ladder); Lane 1: Positive control for Brucella .spp (RB51 vaccine strain); Lane 2: Negative control; Lanes 3, 4: Positive samples for Brucella .spp; Lane 5: Positive control for B. abortus (RB51 vaccine strain); Lane 6: Negative control; Lanes 7, 8: Positive samples for B. abortus.
The highest level of contamination with Brucella was observed in the eastern region of Lorestan province. Out of 24 milk samples, 12 samples (50%) were positive. While in the central, northern, northwestern, southern and western regions of the province, this rate was 2 samples were determined (14.2%), 3 samples (20%), 1 sample (7, 6), 2 samples (14.2 %) and 6 samples (30%), respectively. In addition, the prevalence of B. abortus in the east of the province with 9 samples (75%) was identified more than other areas. The results of this study show that there is a significant difference between the frequency of milk contamination in the eastern regions of Lorestan province with other regions (p <0.05). Therefore, it can be concluded that the geographical area significantly effects on the rate of Brucella infection in Lorestan province (p <0.05) (Table 2).
Table 2. Prevalence of Brucella spp. and B. abortus detection in different geographical areas.
| Total | Negitive | B. abortus | Brucella ssp. | Area |
| 24 14 15 13 14 20 |
12 12 12 12 12 14 |
9 2 2 1 1 4 |
12 2 3 1 2 6 |
East Center North North West South West |
| 100 | 74 | 19 | 26 | Total |
In this study, three age groups (less than 4 years, 4 to 6 years and over 6 years) were selected. From 35 milk samples which were belonged to less than 4 years old group, 11 samples (31.4%) and of 35 milk samples which were taken from the 4 to 6 years group 11 samples (31.4%) were positive for Brucella infection. Also, out of 30 samples from the over 6 years old group, 4 samples (13.3%) were infected with Brucella. The results of this study showed that there was no significant relationship between the presence of Brucella and the age of cattle.
In cattle populations in industrial units, testing and slaughter programs are implemented and comprehensive vaccination of cattle and calves with RB51 and S19 vaccine is also done, so the basis of prevention of brucellosis is its control in animal populations. Despite extensive efforts to combat the disease, prevalence of brucellosis is not only high among the livestock population, but also the occurrence of the disease in the human population is considered as an infectious disease with a higher prevalence than other infectious diseases in most provinces of the country (3,7-11). Awareness of the number of positive cases and the incidence of disease in humans and livestock in the provinces, epidemiological characteristics of infected areas in the two sectors of health and veterinary through the exchange of information in recent years has been one of the main activities in combating the disease which requires more extensive studies in this field.
In a study conducted in 2012 in Kurdistan province by Shafiei et al, using PCR method on 60 samples of raw cow's milk, they found that 20 samples (33.33%) were infected with Brucella bacteria, of which nine samples (45%) were detected to be B. abortus. The author attributes the high prevalence of brucellosis to the proximity of Kurdistan province to countries such as Iraq and Turkey and the entry of non-native strains into Kurdistan province and the possible spread of B. abortus vaccine in milk after vaccination in the studied areas (12). In the study by Khalili et al, which was performed to evaluate the contamination of raw milk with Brucella by PCR method in Kerman, 8.3% of milk samples were positive for the genome of Brucella spp. (13). In another similar study in 2017, in Lorestan province by Shams et al. The prevalence of Brucella in 120 samples of milk tanks was reported to be 10% (14). In the study by Shakrian et al. (2012), in Isfahan and Chaharmahal Bakhtiari provinces, which aimed to investigate the contamination of raw cow milk samples with B. abortus by PCR method, only 1% of the samples were infected with B. abortus. These results are not in accordance with the results of the present study, which may be due to differences in sampling method, number of samples, geographical factors of the study areas, vaccination and measures to control this disease among the livestock of these two provinces. The study of geographical distribution of this disease shows that Chaharmahal and Bakhtiari province are classified in the group with moderate infection and Isfahan province in the group with low pollution, while Lorestan province is in the group with high prevalence (15). In the study by Entezari and Sepahvand, (2014) it has been shown that climatic conditions and geographical environment can be one of the factors affecting the prevalence of the brucellosis disease in susceptible areas (16). In confirmation of this report, the present study also showed that there is a significant difference between the frequency of bovine milk contamination in the eastern regions of Lorestan province with other regions (p <0.05). Also, this study showed the highest prevalence of brucellosis after the eastern region of the province (Aligudarz, Azna and Dorud cities) in the western part of the province (Hennpoldakhtar and Kuhdasht cities). Due to the summer and winter location of the regions of the province, as well as the traffic route of nomadic herds, having suitable livestock pastures, has caused the highest prevalence of brucellosis in these areas. In a comparison of Khalili study in Kerman province and Shams in Lorestan province on samples with the same conditions obtained from milk reservoirs, it was found that the prevalence of brucellosis in Lorestan province is higher than Kerman province. Therefore, it can be concluded that the prevalence of brucellosis is lower in Kerman, Isfahan, Chaharmahal and etc. provinces, than in Lorestan province (13–15) and in parallel, the higher prevalence of this disease in Kurdistan province (12) can be due to the effects of geographical area on the rate of Brucella infection and excretion through milk. In addition, this argument is confirmed by comparing the prevalence of Brucella in the present study with studies conducted abroad. As, The prevalence of Brucella spp. and B. abortus in raw milk in developing countries such as Sudan is reported to be 22.4% and 40% (17), Kenya 18.9% and 65.5%, respectively (18). Also in Iraq, the prevalence of Brucella has been reported from 8.4% to 56% by serological examination (19). While in most European countries brucellosis has been eradicated or has a very low prevalence (20, 21). So far, no study has examined the relationship between the age of the animal and the prevalence of brucellosis. The present study showed that there is no significant relationship between the prevalence of brucellosis and the age of the animal. However, Brucella is more prevalent in cows under 6 years of age than in cattle over 6 years of age. This seems to be due to the vaccine received over many years and the safety of older animals.
The findings of the present study show that the prevalence of brucellosis is still high in Lorestan province and more comprehensive planning, and policies should be done to prevent and eradicate this disease, especially in the eastern and western parts of the province. In addition, due to the high livestock population in these areas and the main role of dairy products in the diet of the people, especially the cheese preparation in rural areas from raw milk, necessary training should be provided to detect the presence of Brucella bacteria in cow's milk and to investigate the possibility of contracting Brucellosis in case of consumption of raw milk and contaminated dairy products by health and veterinary organs.
The authors would like to thank Mr. Ali Karimpor and Veterinary personnel of Noor Abad and Kuhdasht counties for their aid in sample collection.
This research is financially supported by Lorestan University
Conflicts of Interest
The authors declare that they have no conflict of interest.
Received: 2022/04/14 | Accepted: 2022/06/26 | ePublished: 2022/08/8
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








