BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1614-en.html
2- Department of Medicine, Faculty of Medicine, Laval University, Quebec, Canada
3- Zanjan pharmaceutical biotechnology research center, Zanjan University of Medical Sciences, Zanjan, Iran ,
In recent years, hospital-acquired infections (HAIs) have become a global health concern that can lead to higher morbidity and mortality rates, length and costs of hospitalization, and greater use of antibiotics (1). Healthcare-associated infections result in almost 7.1 million annual cases and 99,000 annual deaths in the United States (2). HAIs develop two to three days after patient admission. The symptoms of the related infection should not be observed in the patient at the time of hospitalization, and the relevant disease should not be in the incubation period (3, 4). If nosocomial pathogens are found at a certain body site of an asymptomatic patient, including blood or cerebrospinal fluid, the person might be considered infected. Similarly, healthcare staff may acquire HAIs (5, 6). Almost all microorganisms can cause infections in hospitalized patients; however, very few organisms are considered nosocomial pathogens (7, 8).
Compared with bacteria involved in almost 90% of HAIs, fungi, viruses, mycobacterium, and protozoans are less responsible for nosocomial infections (9, 10). The microorganisms frequently contributing to HAIs include Staphylococcus aureus, Acinetobacter spp., Pseudomonas aeruginosa, Bacillus cereus, Streptococcus spp., enterococci, Legionella, coagulase-negative staphylococci and members of the Enterobacteriaceae family. Based on the data, Escherichia coli, P. aeruginosa, S. aureus and enterococci play an important role (9). As an arising nosocomial bacteria, E. coli is capable of causing severe health problems. It is oxidase negative, a facultatively anaerobic, rod-shaped gram-negative organism that is generally found in the gut of healthy individuals and other animals. A wide range of diseases, including urinary tract infections, pneumonia, neonatal meningitis, gastroenteritis, and septicemia, are caused by this microorganism (11). Due to certain virulence factors such as capsule, sequestration of growth factors, endotoxins, antigenic phase variation, and antimicrobial-resistant some strains of E. coli can cause infection (12). Of major concern is the rise of multidrug-resistant strains of E. coli which have been isolated from the environment, animals, and hospitalized patients worldwide. In particular, the emergence of fluoroquinolones resistant, Carbapenemases, and extended-spectrum β-lactamases producing strains has challenged the infection treatment (13). Thus, the infections caused by this antimicrobial-resistant microorganism are difficult to treat, as the proper antibiotic selection is limited (14). Hospital environments and surfaces naming ceilings, floors, windows, walls, doors, and medical equipment can transmit nosocomial pathogens to patients (15). Hence, decontaminating, including sterilization as well as applying disinfectants, is crucial to reduce the microbial spread and cross-infection risk. Common disinfectants include glutaraldehyde, sodium hypochlorite, phenolic compounds, iodophors, 70% ethyl alcohol and 92% isopropyl alcohol, peracetic acid/ hydrogen peroxide (0.5 to 2%) in addition to sodium hypochlorite (1%), quaternary ammonium compounds and a chlorhexidine (16). However, selecting the right disinfectant is challenging since a broad range of products is available on the market. In addition, various nosocomial pathogens show different susceptibility patterns to disinfectants (17). It has also been shown that these microorganisms have a high resistance to the lethal effects of disinfectants and can grow inside some of them, which is the cause of infection transmission and cross-infection in medical centers (18). Therefore, it is essential to identify microorganisms and evaluate microbial sensitivity to the available disinfectants by various techniques. The aim of the present study was to determine susceptibility of isolated E. coli strains from two university hospitals in Zanjan, Ayatollah Mousavi and Hazrat Valiasr hospitals, to seven widely used disinfectants which are routinely applied for disinfection of surfaces, floors and facilities both in presence and without organic substances.
Bacterial Isolation and Identification
One hundred clinical strains of E. coli were randomly selected and isolated from hospitalized patients and outpatients at Ayatollah Mousavi and Hazrat Valiasr hospitals of Zanjan city of Iran from June 2019 to November 2020. The isolated E. coli were further identified on selective media of Eosin Methylene Blue agar (19).
Disinfectants Selection
Aniosyme DD1 0.5%, Steranios 2%, Aniospray 29 and Surfanios 0.5% at Valiasr hospital and PROSEPT® Floor 0.75%, PROSEPT® Instru 0.05% and PROSEPT® Med at Ayatollah Mousavi hospital were used as selected disinfectants to test the in vitro susceptibility of E. coli isolates. Table 1 shows the compositions and final concentrations according to the manufacturer's literature.
Table 1. Composition, application and preparation of disinfectants used at Hazrat Valiasr and Ayatolah Musavi hospital
| Disinfectant | Composition in 100 g | Applications | Preparation |
| Aniosyme DD1 | - Quaternary ammonium propionate - Polyhexamethylene biguanide hydrochloride - Enzymatic complex - Surface-active agents - Stabilising agents - Sequestrating agents |
- Reinforced pre-disinfection and cleaning of instruments - Cleaning in ultrasonic trays - Collection of contaminated instruments |
0.5% (Pour a 25 ml dose in 5 liters of cold or tepid water) |
| Steranios 2% | - 2% glutaraldehyde, buffered at pH 6 in the presence of surface effects catalysor - STERANIOS 2% NG and STERANIOS 2% ECS contain two compounds limiting glutaraldehyde evaporation, when associated |
- Medical devices - Surgical, medical, endoscopic - Heat-sensitive equipment |
Ready-to-use |
| Aniospray 29 | - Hydroalcoholic solution (ethanol 55%) - Quaternary ammonium propionate - Fragrance |
- Previously cleaned, non-immersible medical devices resistant to alcohol (stethoscopes, cables and connectors, pressure sensors, blood sugar testers,…) | Ready-to-use |
| Surfanios | - N-(3-aminopropyl)-N-dodecylpropane-1,3-diamine - Didecyldimethylammonium chloride - Excipients |
- Floors - Walls - Medical equipment and non-invasive medical devices |
0.0025 (20ml for 8L) |
| PROSEPT® Floor | - 10 g quaternary ammonium compounds - Perfumes - Dyes |
- Large surfaces of medical devices | 0.75% |
| PROSEPT® Instru | - 9.9 g of alkylamine - 3.6 g of dialkyldimethylammonium chloride - Cleaning booster - Auxiliaries |
- Mirrors - Polishers - Instruments - Silicone parts - Plastic spatulas - Acrylic glass slabs |
0.5 % |
| PROSEPT® Med | - 44.9 g isopropanol - 30.0 g 1-propanol - 0.15 g dialkyldimethylammonium chloride - Water - Natural moisturizing factors - Perfume |
Surgical and hygienic hand and forearm | Ready-to-use |
Disinfectants Susceptibility Assay
The disinfectant susceptibility of E. coli was determined using a slightly modified microdilution method suggested by NCCLS (National Committee for Clinical Laboratory Standards) guidelines (20). To perform the technique, ninety-six wells of a microtiter plate were filled with 100 µL freshly prepared Mueller Hinton Broth (MHB). Next, the columns were filled with 100 µL 0.5 McFarland adjusted microbial suspension and 100 µL disinfectant dilution sequentially from the lowest to the highest dilution of the biocide agent. The last two columns were used as growth control (100 µL MHB + 100 µL bacterial suspension) and sterile control (200 µL MHB), respectively. The microtiter plate was incubated at 37°C for 24h and visually examined for any turbidity. The lowest concentration of the disinfectant that inhibited bacterial growth, causing no turbidity, is considered a minimum inhibitory concentration (MIC). 10 µL suspension from clear wells was sub-cultured on the surface of Mueller Hinton Agar (MHA) and incubated at 37°C for 24h to determine the minimum bactericidal concentration (MBC). The efficacy of disinfectants can be influenced by the presence of organic materials. Disinfectants may be inactivated or blocked from attaching microbial membrane receptors by interfering with organic materials (21-23). An actual dirty situation was mimicked by adding 0.5% w/v of bovine serum albumin (BSA) into intended wells, determining MIC and MBC to examine the influence of proteins on the disinfection process. The tests were performed in triplicate (24).
MIC, MICal, MBC, and MBCal Values of Hospital Disinfectants
One hundred E. coli samples were isolated from Hazrate Valiasr and Ayatollah Mousavi university hospitals, identified by inoculation onto EMB agar based on microbial laboratory methods. MIC and MBC (without bovine serum albumin), MICal and MBCal (with bovine serum albumin) of seven commonly used hospital biocides against clinical isolates of E. coli were evaluated.
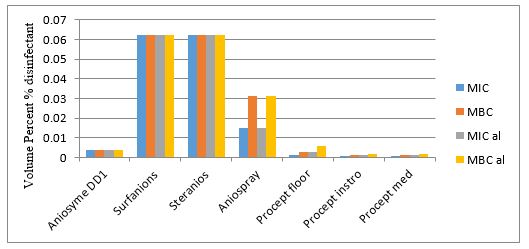
The obtained values are shown in Table 2. PROSEPT® Med and Aniospray 29 showed the lowest mean MIC values at 0.263 × 10-2 and 0.504 × 10-2 v/v %, respectively. According to Table 2, bacteriostatic activities of other biocides are classified as follows; PROSEPT® Instru> Aniosyme DD1> PROSEPT® Floor> Surfanios> Steranios. Furthermore, antibacterial properties were studied under dirty conditions. In the presence of BSA, higher MIC values of Aniosyme DD1, Surfanios, Steranios 2%, Aniospray 29, PROSEPT® Floor, and PROSEPT® Med were obtained, representing a reduction in the bacteriostatic activities. The MICal values of PROSEPT® Instru did not exhibit any difference; while, that of Steranios 2% had experienced about a four-fold increase (from 13.637 × 10-2 to 61.75 × 10-2 v/v %). As shown in Table 2, PROSEPT® Med and Aniospray 29 had the highest bactericidal activities with MBC values at 0.419 × 10-2 and 0.728 × 10-2 v/v %, respectively. The bactericidal potency of the remaining biocides is as follows; PROSEPT® Instru> Aniosyme DD1> PROSEPT® Floor> Surfanios> Steranios. To simulate the so-called 'dirty' situations, MBCal values were also measured. In the presence of BSA, higher MBC values of Surfanios, Steranios 2%, and PROSEPT® Instru were obtained. Antibacterial efficiency of Aniosyme DD1, Aniospray 29, and PROSEPT® Floor did not alter in the stimulated dirty condition, and MBCal values of PROSEPT® Med were reduced (Table 2).
Table 2. Median and mean MIC, MBC, MICal and MBCal values (v/v %) of selected disinfectants against E. coli isolates, the data shown are multiplied in 102
| MIC | MBC | MICal | MBCal | ||||||||
| Disinfectant | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | |||
| Aniosyme DD1 | 0.78 | 1.04 ± 0.22 | 1.5 | 1.56 ±0.52 | 1.5 | 1.64 ± 0.51 | 1.5 | 1.469 ± 0.523 | |||
| Surfanios | 1.5 | 1.283 ± 0.54 | 1.5 | 2.03 ± 0.49 | 1.5 | 1.8 ± 0.50 | 1.5 | 2.478 ± 0.48 | |||
| Steranios 2% | 12 | 13.637 ± 3.9 | 12 | 15.67 ± 1.05 | 12 | 61.75 ± 0.44 | 12 | 20.7 ± 3.425 | |||
| Aniospray 29 | 0.39 | 0.504 ± 0.28 | 0.39 | 0.73 ± 0.19 | 0.78 | 0.655 ± 0.12 | 0.78 | 0.727 ± 0.258 | |||
| PROSEPT® Floor | 1.1 | 1.27 ± 0.56 | 1.1 | 1.74 ± 0.51 | 1.1 | 1.595 ± 0.52 | 1.1 | 1.635 ± 0.518 | |||
| PROSEPT® Instru | 0.78 | 0.72 ± 0.14 | 0.78 | 1.08 ± 0.26 | 0.39 | 0.696 ± 0.27 | 0.78 | 1.064 ± 0.573 | |||
| PROSEPT® Med | 0.19 | 0.263 ± 0.129 | 0.39 | 0.419 ± 0.295 | 0.39 | 0.435 ± 0.05 | 0.19 | 0.226 ± 0.027 | |||
Minimum Inhibitory Concentration (MIC)
Minimum Bactericidal Concentration (MBC)
Minimum Inhibitory Concentration in the presence of bovine serum albumin (MICal)
Minimum Bactericidal Concentration in the presence of bovine serum albumin (MBCal)
MICs and MICals distribution of disinfectants are given in Tables 3 and 4. PROSEPT® Instru and PROSEPT® Med had the lowest MIC values, inhibiting 4 and 1 isolates in order, while other disinfectants did not show antibacterial effects at this concentration (0.048 × 10-2 v/v %). Interestingly, Steranios 2% demonstrated the highest MIC value at 50 × 10-2 v/v %. By comparing these tables, it can be deduced that the presence of BSA generally decreased the antibacterial activities of the biocides.
Also, distributions of MBCs and MBCals are presented in Tables 3 and 4. As shown in Table 3, the lowest MBC values belong to PROSEPT® Instru and PROSEPT® Med, which killed 8 and 5 isolates respectively at 0.097 × 10-2 v/v %, showing their potent bactericidal activities. This is while Steranios 2% tended to have the highest MBC amount (50 × 10-2 v/v %). As tables 2 illustrated, adding BSA to the wells raised the median MBCs of Aniospray 29 (from 0.39 × 10-2 to 0.78 × 10-2 v/v %); whilst, that of PROSEPT® Med decreased from 0.39 × 10-2 to 0.19 × 10-2 v/v %. The median MBCs of the other biocides didn't change; however, a decrease in their bactericidal properties is evident (Tables 3 and 4).
Table 3. Distribution of MIC (%), MBC (%) and MBCal (%) of various disinfectants by microtiter method
| disinfectant | Number of strains at each MIC (%) of disinfectant | ||||||||||||
| 0.5×10-1 | 2.5×10-1 | 1.2×10-1 | 6.25×10-2 | 3.1×10-2 | 1.5×10-2 | 7.8×10-3 | 3.9×10-3 | 1.9×10-3 | 9.7×10-4 | 4.8×10-4 | 2.4×10-4 | ||
| AnDD1 | 0 | 0 | 0 | 0 | 6 | 27 | 48 | 19 | 0 | 0 | 0 | 0 | |
| Surf. | 0 | 0 | 0 | 1 | 7 | 43 | 43 | 6 | 0 | 0 | 0 | 0 | |
| Stera. | 1 | 11 | 85 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anspy | 0 | 0 | 0 | 0 | 3 | 4 | 15 | 43 | 35 | 0 | 0 | 0 | |
| Pinstro | 0 | 0 | 0 | 0 | 0 | 26 | 27 | 24 | 8 | 11 | 4 | 0 | |
| Pmed | 0 | 0 | 0 | 0 | 0 | 1 | 6 | 22 | 51 | 19 | 1 | 0 | |
Continuation of Table 3 from the previous page. Distribution of MIC (%), MBC (%) and MBCal (%) of various disinfectants by microtiter method
| disinfectant | Number of strains at each MBC (%) of disinfectant | |||||||||||||||||||||||
| 0.5×10-1 | 2.5×10-1 | 1.2×10-1 | 6.25×10-2 | 3.1×10-2 | 1.5×10-2 | 7.8×10-3 | 3.9×10-3 | 1.9×10-3 | 9.7×10-4 | 4.8×10-4 | 2.4×10-4 | |||||||||||||
| AnDD1 | 0 | 0 | 0 | 2 | 18 | 40 | 32 | 8 | 0 | 0 | 0 | 0 | ||||||||||||
| Surf. | 0 | 0 | 0 | 7 | 33 | 47 | 21 | 2 | 0 | 0 | 0 | 0 | ||||||||||||
| Stera. | 4 | 17 | 78 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| Anspy | 0 | 0 | 0 | 0 | 5 | 12 | 23 | 50 | 10 | 0 | 0 | 0 | ||||||||||||
| Pinstro | 0 | 0 | 0 | 0 | 10 | 31 | 30 | 12 | 9 | 8 | 0 | 0 | ||||||||||||
| Pmed | 0 | 0 | 0 | 0 | 0 | 5 | 18 | 31 | 41 | 5 | 0 | 0 | ||||||||||||
| Disinfectant | Number of strains at each MBC al (%) of disinfectant | |||||||||||||||
| 0.5×10-1 | 2.5×10-1 | 1.2×10-1 | 6.25×10-2 | 3.1×10-2 | 1.5×10-2 | 7.8×10-3 | 3.9×10-3 | 1.9×10-3 | 9.7×10-4 | 4.8×10-4 | 2.4×10-4 | |||||
| AnDD1 | 0 | 0 | 0 | 4 | 4 | 20 | 20 | 12 | 0 | 0 | 0 | 0 | ||||
| Surf. | 0 | 0 | 0 | 8 | 12 | 44 | 32 | 4 | 0 | 0 | 0 | 0 | ||||
| Stera. | 12 | 16 | 60 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Anspy | 0 | 0 | 0 | 0 | 0 | 12 | 43 | 31 | 8 | 4 | 0 | 0 | ||||
| Pinstro | 0 | 0 | 0 | 0 | 4 | 12 | 32 | 24 | 24 | 4 | 0 | 0 | ||||
| Pmed | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 36 | 0 | 0 | 0 | |||||
Aniosyme DD1 (AnDD1), Surfanios (Surf.), Steranios (Stera), Aniospray (Anspy), Procept instro (Pinstro), Procept med (Pmed) Procept floor (Pfloor)
Table 4. Distribution of MIC (%), MBC (%) and MBCal (%) of Procept floor by microtiter method
| disinfectant | Number of strains at each MIC (%) of disinfectant | |||||||||||||||||||||||||
| 8.1×10-1 | 3.9×10-2 | 6.4×10-2 | 3.2×10-2 | 1.1×10-2 | 8.5×10-3 | 9.2×10-3 | 4.1×10-3 | 3.7×10-4 | 6.3×10-1 | |||||||||||||||||
| Pfloor | 0 | 0 | 3 | 5 | 46 | 44 | 1 | 1 | 0 | 0 | ||||||||||||||||
| disinfectant | Number of strains at each MBC (%) of disinfectant | |||||||||||||||||||||||||
| 8.1×10-1 | 3.9×10-2 | 6.4×10-2 | 3.2×10-2 | 1.1×10-2 | 8.5×10-3 | 9.2×10-3 | 4.1×10-3 | 3.7×10-4 | 6.3×10-1 | |||||||||||||||||
| Pfloor | 0 | 0 | 7 | 15 | 53 | 23 | 2 | 0 | 0 | 0 | ||||||||||||||||
| disinfectant | Number of strains at each MBC al (%) of disinfectant | |||||||||||||||||||||||||
| 8.1×10-1 | 3.9×10-2 | 6.4×10-2 | 3.2×10-2 | 1.1×10-2 | 8.5×10-3 | 9.2×10-3 | 4.1×10-3 | 3.7×10-4 | 6.3×10-1 | |||||||||||||||||
| Pfloor | 0 | 0 | 4 | 16 | 51 | 29 | 0 | 0 | 0 | 0 | ||||||||||||||||
Figure 1. The investigated criteria of MIC, MBC, MICal, MBCal for hospital disinfectants against E. coli isolates
Sample-hospital
| Sample-hospital | Source | Sample-hospital | Source | ||
| 1 | E. coli 1M | catheter | 48 | E. coli 13V | wound |
| 2 | E. coli 2M | bedsore | 49 | E. coli 12V | bedsore |
| 3 | E. coli 3M | blood | 50 | E. coli 13V | UTI |
| 4 | E. coli 4M | catheter | 51 | E. coli 14V | catheter |
| 5 | E. coli 5M | catheter | 52 | E. coli 15V | catheter |
| 6 | E. coli 6M | sepsis | 53 | E. coli 16V | catheter |
| 7 | E. coli 7M | catheter | 54 | E. coli 17V | wound |
| 8 | E. coli 8M | UTI | 55 | E. coli 18V | UTI |
| 9 | E. coli 9M | UTI | 56 | E. coli 19V | bedsore |
| 10 | E. coli 10M | UTI | 57 | E. coli 20V | UTI |
| 11 | E. coli 11M | UTI | 58 | E. coli 21V | UTI |
| 12 | E. coli 12M | UTI | 59 | E. coli 22V | UTI |
| 13 | E. coli 13M | blood | 60 | E. coli 23V | UTI |
| 14 | E. coli 14M | UTI | 61 | E. coli 24V | UTI |
| 15 | E. coli 15M | sepsis | 62 | E. coli 25V | sepsis |
| 16 | E. coli 16M | - | 63 | E. coli 26V | UTI |
| 17 | E. coli 17M | bedsore | 64 | E. coli 27V | catheter |
| 18 | E. coli 18M | bedsore | 65 | E. coli 28V | catheter |
| 19 | E. coli 19M | UTI | 66 | E. coli 29V | catheter |
| 20 | E. coli 20M | UTI | 67 | E. coli 30V | catheter |
| 21 | E. coli 21M | UTI | 68 | E. coli 31V | catheter |
| 22 | E. coli 22M | UTI | 69 | E. coli 32V | catheter |
| 23 | E. coli 23M | UTI | 70 | E. coli 33V | catheter |
| 24 | E. coli 24M | catheter | 71 | E. coli 34V | catheter |
| 25 | E. coli 25M | catheter | 72 | E. coli 35V | wound |
| 26 | E. coli 26M | catheter | 73 | E. coli 36V | UTI |
| 27 | E. coli 27M | catheter | 74 | E. coli 37V | UTI |
| 28 | E. coli 28M | catheter | 75 | E. coli 38V | wound |
| 29 | E. coli 29M | UTI | 76 | E. coli 39V | wound |
| 30 | E. coli 30M | - | 77 | E. coli 40V | meningitis |
| 31 | E. coli 31M | bedsore | 78 | E. coli 41V | sepsis |
| 32 | E. coli 32M | UTI | 79 | E. coli 42V | UTI |
| 33 | E. coli 33M | - | 80 | E. coli 43V | bedsore |
| 34 | E. coli 34M | catheter | 81 | E. coli 44V | catheter |
| 35 | E. coli 35M | catheter | 82 | E. coli 45V | - |
| 36 | E. coli 1V | meningitis | 83 | E. coli 46V | bedsore |
| 37 | E. coli 2V | - | 84 | E. coli 47V | UTI |
| 38 | E. coli 3V | bedsore | 85 | E. coli 48V | UTI |
| 39 | E. coli 4V | sepsis | 86 | E. coli 49V | catheter |
| 40 | E. coli 5V | UTI | 87 | E. coli 50V | catheter |
| 41 | E. coli 6V | UTI | 88 | E. coli 51V | catheter |
| 42 | E. coli 7V | UTI | 89 | E. coli 52V | catheter |
| 43 | E. coli 8V | bedsore | 90 | E. coli 53V | UTI |
| 44 | E. coli 9V | UTI | 91 | E. coli 54V | sepsis |
| 45 | E. coli 10V | UTI | 92 | E. coli 55V | UTI |
| 46 | E. coli 11V | blood | 93 | E. coli 56V | UTI |
| 47 | E. coli 12V | UTI |
Various factors such as the biological fluids of patients and contaminated appliances can cause contamination of hospital environments, increasing the prevalence of HAIs. Thus, it is essential to select not only the most potent but also efficient disinfectants to control and prevent the spreading of nosocomial infections (25-27). This study aimed to investigate the antimicrobial efficacy of seven selected disinfectants used at Hazrat Valiasr and Ayatollah Mousavi hospitals against 100 isolates of E. coli according to MIC, MBC, MICal, and MBCal values. The obtained data revealed that selected disinfectants might have a different range of antimicrobial activities and different effects against isolated microorganisms. Hence, it is crucial to evaluate the MIC and MBC values of applied disinfectants to determine their effectiveness against nosocomial pathogens.
Based on our findings, PROSEPT® Med had the lowest values of MIC and MBC, followed by Aniospray 29 and PROSEPT® Instru in order. This is while those of Steranios 2% were the highest values with the widest range. In contradiction to our findings, Nabizadeh reported the lowest MIC and MBC values of Steranios 2%, which were determined by the microdilution method. This is probably due to the different selection of disinfectants: Steranios 2%, Deconex HLDPA, and Microzed Quatenol, along with different bacterial cell structures of tested microorganisms (Enterococcus faecalis and Burkholderia cepacia) (17). To the best of our knowledge, antimicrobial activities of PROSEPT® Med, Aniospray 29, and PROSEPT® Instru against E. coli were not studied; however, disinfectant susceptibility of E. coli was reported by some authors. Xia and coworkers studied the susceptibility of 510 collected E. coli isolates against cetyltrimethylammonium bromide, cetylpyridinium chloride, CHX, benzalkonium chloride, and triclosan. The MICs of the five disinfectants were determined using the agar dilution method, and CHX showed the lowest MIC values, while triclosan had the widest range of MICs (28). In another similar research by Oosterik, susceptibility of a selection of 97 E. coli isolates to various antibiotics, and the five most applied disinfectants in the poultry industry were determined. MIC and MBC values of glyoxal, formaldehyde, hydrogen peroxide, glutaraldehyde, and a quaternary ammonium compound were reported as concentration ranges by the microbroth dilution assay. The results revealed that alkyldimethylbenzylammonium chloride had the lowest MIC and MBC values. In addition, phenotypic resistance to the disinfectants was not observed, while tested antibiotics were selected for resistance to E. coli (29, 30). Oosterik's findings correlate well with our results, demonstrating that disinfectants containing quaternary ammonium compounds (PROSEPT® Instru, PROSEPT® Med, Aniospray 29, PROSEPT® Floor, and Surfanios) had greater antimicrobial effects against E. coli isolates (31). The physical and ionic stability of bacterial membrane can be disrupted by quaternary ammonium compounds (32).
Since organic materials are present in the bodies of the patient and hospital environments that inhabit nosocomial pathogens such as E. coli, their effects on the antimicrobial properties of biocides should be taken into account (24). Thus, this study was extended to evaluate the reduction effects of BSA as an organic material on the antimicrobial efficiency of the disinfectants. With the exception of the MIC value of PROSEPT® Instru, which nearly stayed the same in the presence of BSA, those of Aniosyme DD1, Surfanios, Steranios 2%, Aniospray 29, PROSEPT® Floor, and PROSEPT® Med experienced an increase of 6.44%, 68.3%, 351.81%, 29.96%, 29.15%, and 65.39% respectively. Considering bactericidal effects of the disinfectants, BSA increased MBC amounts of Surfanios, Steranios 2%, and PROSEPT® Instru to 39.89%, 32.08%, and 19.81%, respectively. However, those of Aniosyme DD1, Aniospray 29, and PROSEPT® Floor did not show a significant alteration, and MBC of PROSEPT® Med reduced to 40.06%. These results agree with the study conducted by Vickery. The study investigated antibacterial activities of hydrogen peroxide (Oxivir and 6% H2O2 solution), peracetic acid (Surfex and Proxitane), and chlorine (Chlorclean and sodium dichloroisocyanurate tablets) against S. aureus dry-surface biofilm with and without organic soil (5% bovine calf serum (BCS) and 10% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (33). The findings showed that the presence of organic material highly affected bactericidal efficacies of Proxitane, Oxivir, Chlorclean, sodium dichloroisocyanurate tablets, and H2O2 solution, whereas those of Surfex did not change (34).
This study was performed to determine the lethal concentrations of clinically used disinfectants. In conclusion, PROSEPT® Med was the most effective biocide against E. coli followed by Aniospray 29 and PROSEPT® Instru. Generally, the disinfectants used at Ayatollah Mousavi hospital showed higher antibacterial effects than those used at Hazrat Valiasr hospital. Furthermore, our findings suggest that the presence of organic materials, including exudation and blood, may reduce disinfectants' efficacy, increasing the survival rate of microbes. Thus, the susceptibility profile of nosocomial microbes against biocides should be monitored in the presence and absence of organic materials routinely in medical centers.
We would like to appreciate the cooperation of the Hazrat Valiasr and Ayatollah Mousavi hospitals that submitted the microbial isolates to the microbiology laboratory at the Faculty of Pharmacy.
All authors participated in conducting the project and approval the final manuscript.
The study was approved by the ethics committees of the Zanjan University of Medical sciences. Ethical Code: ZUMS.REC.1394.280.
This study was extracted from the Pharm-D thesis and supported by the School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran.
Conflicts of Interest
The authors have no conflict of interest to declare relevant to this article's content.
Received: 2022/01/5 | Accepted: 2022/05/17 | ePublished: 2022/08/8
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |