BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1497-en.html
2- Institute of Microbiology, University of Agriculture, Faisalabad, Pakistan ,
3- Department of Parasitology, University of Agriculture, Faisalabad, Pakistan
For the good health of people, a balanced diet is required. A vital component, protein, plays an essential role in structure development in a balanced diet. Animals and Plants are primary sources of proteins. The essential protein requirement is 102.7 g per day per person, and approximately 66% of people in Pakistan are deficient in protein. To overcome the high demand for protein, the poultry industry contributes significantly to livestock production (1, 2). the poultry industry has a short productive span, reproductive traits, distribution throughout the world, and egg production favors Poultry as a significant protein source. The poultry industry plays a vital role in economic development and alleviation of poverty in Pakistan, with around 33,146 registered poultry farms with 89.4 million poultry birds, among which the number of layers birds is 16.9 million, broilers 70.7 million, and breeder flock comprises 17.5 million. Poultry is one of Pakistan's largest sectors, contributing around 1.3% to its national GDP.
In the poultry industry, immunosuppression is a persistent problem, and its graph increases with intensive commercial farming. Viruses primarily infect and destroy immune cells and are the leading cause of immunosuppression. The factors that lowered the production and expected growth in the immunosuppressive flock are chronic disease situations, suboptimal response to a vaccine and susceptibility to opportunistic pathogens. Immunosuppressive viruses have economic and societal effects. Besides this, antibiotic resistance and drug residues in meat are also a significant threat to human health (3).
IBD is characterized by destroying the Bursa of Fabricius (Primary lymphoid organ), where B cells mature and differentiate. The virus is very stable and resistant to physical and chemical agents, heat and ultraviolet radiation. Thus, it persists in poultry houses for several weeks after cleaning and disinfection (4, 5). No specific treatment is available against IBDV, but palliative treatment is a practice to control this disease. Principle means administering killed and attenuated vaccines to control IBD (6). A recent immune complex vaccine against infectious bursal disease virus has been released to replace the conventional vaccine.
The immune complex (Icx) vaccine was initially developed by combining hyperimmune chicken serum raised against IBDV and the Live intermediate plus IBD virus vaccine. The infectivity of the native IBDV and ICx vaccine was investigated in terms of the replication rate and difference of target organs. In the present study, the modification of the immune complex vaccine was suggested where chicken egg yolk antibodies (IgY) mixed with live IBD virus-based immune complex vaccine is the way to advance the therapeutic potential of the vaccine. This study aims to develop a modified immune complex vaccine comprising chicken egg yolk (IgY) antibodies mixed with live, attenuated IBD virus. The study was designed to evaluate the potential of IgY-based immune complex vaccine for controlling infectious bursal disease in poultry birds.
IBD Virus Antigen
Infected Bursa of Fabricius (BF) was collected from different diseased commercial farms in the Faisalabad District. During 2019-2020, 30 Bursae were obtained from five IBD suspected outbreaks in Faisalabad, with 10-18% mortality observed at these farms. The infected birds were manifested by inflammation of BF and showed pathognomonic PM lesions. The BF samples were transported to the Molecular Research Laboratory, Institute of Microbiology, the University of Agriculture Faisalabad for molecular diagnosis and characterization after approval from the Institutional Biosafety Committee (IBC), UAF (7). The Bursae of Fabricius were homogenized in a mortar and pestle with phosphate-buffered saline supplemented with Kanamycin (700 µg/mL). To eliminate the cell debris, samples were clarified by centrifugation at 8000 rpm for five minutes. Total RNA was extracted using 200 µL of the supernatant using GeneJet viral purification kit (Thermo Fisher) according to the manufacturer's protocol. SuperScript™ reverse transcriptase kit (Invitrogen, USA) was used for RT-PCR reaction (8).
Immunization of Layer birds with IBD Virus
Ten White Leghorn hens (layers) were housed in an animal house facility, Institute of Microbiology, University of Agriculture Faisalabad, Pakistan. After permission from Institutional Biosafety Committee (UAF), the layer birds were injected with the preparation containing 0.5mL of the inactivated antigen subcutaneously to develop IgY antibodies. The development of egg yolk IgY antibodies was confirmed through the agar gel precipitation method (AGPT). IgY was separated using the Ammonium Sulphate Precipitation method described by Naveed et al. 2018. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to identify the IgY protein. The experiment was performed with a discontinuous buffer system at the Nuclear Institute of Agriculture and Biology (NIAB), Faisalabad. In the SDS-PAGE buffer system, IgY protein was denatured by heating in a buffer containing SDS and 2-mercaptoethanol (ME) (9).
Determination of PFU of IBDV
The IBD virus was adapted in chicken embryo fibroblast (CEF) cells cultured in a 25cm2 flask and incubated at 39 ℃ under 5% CO2 for four days. Cells were monitored for cytopathic effects every 24 hours to determine the plaque-forming unit (PFU). The culture medium was centrifuged at 1800 x g for 10 minutes at 4 ℃, and the supernatant was collected. The supernatant was divided into aliquots and stored at -20 ℃ for virus stock (10).
Titration by Quantal Assay
The cell culture-derived IBD virus was 10-fold diluted in PBS containing antibodies and inoculated (0.1mL) in 10-day old SPF eggs via the CAM route. The embryo was candled every 6 hours post-infection for mortality. After 48 hours post-infection, eggs were chilled at 4 ℃ and observed for lesions (11).
Pathogenesis Studies
Poultry birds (n=10) in 10 groups will be kept at animal house facilities at the Institute of Microbiology, University of Agriculture Faisalabad. After the acclimation period of 24 hours, each group was inoculated with a ten-fold diluted virus. These birds were bled on days 2,4,6,10, and 14 post-infection. After the postmortem, the lymphoid tissues (Bursa, Spleen, Thymus) were collected for gross pathological changes and microscopic examination (10).
Preparation of Immune complex Antigen (Icx)
The immune complex vaccine was prepared by mixing the known quantity of IBD classical live virus in conjunction with IgY chicken antibodies raised in eggs recommended for injection of chickens at day one of age. An equal volume of antigen and antibodies were prepared in 1:1, mixed thoroughly to prepare the immune complex vaccine and stored at a temperature below 10 ℃ for further use.
Sterility and Safety of Immune Complex Vaccine
The prepared immune complex antigen was analyzed for its sterility and safety. Blood agar media and thioglycollate broth medium were aseptically inoculated (0.5 mL) with Icx antigen suspension and incubated for 72 hours at 37 ℃ in an incubator. Rabbits were immunized through a subcutaneous route and were observed for seven days post-immunization for safety studies.
Study Design
Eighty semi-SPF chicks were divided into four groups (twenty in each) and were kept at the animal house facility, Institute of Microbiology, University of Agriculture, Faisalabad. Group-I was kept as an un-inoculated control group. Group-II was injected with a single dose of 0.2 mL of the immune complex vaccine through a subcutaneous route. Group-III was injected with 0.2 ml of the immune complex vaccine on day one, and a booster dose of 0.2 mL was injected after one week. Group-IV was injected with 0.2 mL of the commercially available IBDV live vaccine, followed by the sera collection for six weeks to determine the antibody titers. Immunization titers were measured and statistically analyzed to determine the vaccine performance in birds compared to the commercially available vaccine (12).
Serology
The viral challenge was conducted on the 21st day of age using EID50 /1 mL of infectious bursal disease virus via the oral and ocular routes to three immunized groups of poultry birds. Group-II and III were immunized with the immune complex vaccine. Group IV was immunized with commercial IBD live vaccine, and group-I was kept as a control group. The animals were observed for signs and symptoms of the disease, Bursa to body weight ratio, and postmortem examination for the pathological changes in the Bursa. Collected chicken sera were checked for IBD-specific antibodies using an I-ELISA kit (IDEXX-USA) according to the manufacturer's protocols (12).
Statistical Analysis
The difference in the body weights, bursal body weight ratio (bursal weight/bodyweight x 100), and mean of the antibody titers of the birds used in the experimental trial were tested through factorial analysis (13).
Pathogenesis of IBDV
Although virus apoptosis was detected in the liver and kidneys, extensive replication of the infectious bursal disease virus occurred in the Bursa of Fabricius. The clinical signs included ruffled feathers, anorexia, diarrhea, depression, and the death of the birds. The morbidity was 100% in all infected flocks, while the mortality was 10-18%. The postmortem findings included; Bursal atrophy and lesions, hemorrhages on the thigh and pectoral muscles, and darkened discoloration of pectoral muscles. A yellowish gelatinous material was observed covering the Bursa's external surface with multiple hemorrhages and necrosis areas.
Purification of Egg Yolk Antibodies (IgY)
Egg yolk antibodies (IgY) purified through ammonium sulfate precipitation were analyzed on 10% SDS-PAGE gel under non-reducing and reducing conditions. Analysis showed the optimal precipitation results of the immunoglobulin-Y and exhibited protein bands of 115 kDa (heavy chain) and 30 kDa (light chain).

Figure 1. SDS-PAGE profiling of fragment antibody binding components following the digestion of IgY after staining with Coomassie brilliant blue
Determination of PFU of IBD Virus
The infectious bursal disease virus was adapted to Chicken Embryo Fibroblast cells and the cytopathic effect of the virus was notable four days after the inoculation. The appearance of tiny refractile round cells 24 hours later spread to the entire culture and detached from the dish, leaving empty areas. After overlaying the infected CEF culture with an agar medium, well-defined plaques of 4mm in diameter were developed.
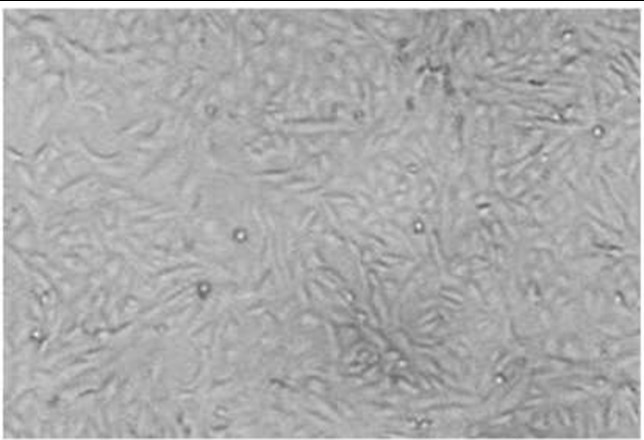
Figure 2. CPE is characterized by round infected cells in CEF cell monolayer infected with IBD virus
Pathogenesis studies in birds
On necropsy, the characterized Bursa lesions were marked atrophy on the 4th day post-inoculation. The pronounced enlargement of the Bursa of Fabricius and occasional haemorrhages with the accumulation of yellowish exudate and longitudinal striations were observed in the infected birds. On day six of the infection, atrophy of the Bursa of Fabricius was clearly seen until the end of the study. The microscopic evaluation of the spleen, thymus and Bursa revealed the lesion only in the Bursa of Fabricius.
Sterility and safety studies of Icx Antigen
The culture media with a suspension of immune complex indicated sterile preparations after 72 hours post-incubation, and no turbidity was observed in the medium. Similarly, the inoculation of Icx on blood agar medium plates was also negative for any growth of the bacteria. The rabbits were observed for one-week post-immunization for safety studies of the immune complex antigen. The change in the body temperature of the rabbits was observed to be normal (101 ± 0.3 ℉ to 103 ± 0.3 ℉), and no mortality was recorded. Other than the mild signs of inflammation at the injection site, the remaining parameters were in the normal range. The swelling from the inoculation was resolved after 24 h.
Comparative Immune Response of Immune Complex Vaccine and Commercial IBD Vaccine in Chicken
The I-ELISA antibody titers in the vaccinated Icx group (G-III) were relatively higher on days 14 and 21 than in the commercially available live IBD vaccinated group. However, these groups showed no obvious difference at 28 days of age, but at day 35, the antibody titer decay was faster in the commercial vaccinated group than in immune complex vaccinated birds. On day 35, the antibody titers of chickens in G-III were significantly higher than in the other groups (P ≤ 0.05) (Figure 3).
Bursa to bodyweight ratio is more indicative of the protection afforded by different vaccines. All the vaccinated nonchallenged groups (G-II, G-III, G-IV) showed higher BB ratios than the control group. The BB ratio was significantly lower in the G-III (Icx) than in the challenge control group (G-I) at 35 days of age (Table 1 & Table 2).
No Bursal changes were observed at day 21 of age in nonchallenged Icx groups (G-II, G-III) and commercial vaccination group (G-IV) except for slight B-cell proliferation in the cortical medullary cells in G-IV. Similarly, no noticeable changes were observed in G-II, III, and IV upon challenge. The G-I (challenge control) showed massive degenerative changes, apoptosis, infiltration of inflammatory cells and central necrosis. On the 28th day, G-II and III showed a well-formed healthy epithelial lining of the pica and characteristic cortical lymphocytes were highly activated. G-IV showed multiple immune reactive lymphoid follicles and mild thickening of the interstitial connective tissue by fibroblastic proliferation. The challenge control G-I showed massive central necrosis, heterophilic infiltration, apoptosis, and the disappearance of lymphoid cells and the necrotic cortical layer (Figure 4).
All the vaccinated groups (G-II, G-III and G-IV) were protected against the infectious bursal disease virus challenge. In comparison, the G-I group (Control) birds showed 60% mortality on days 4th and 5th post-challenge. The G-I challenged control group showed IBD lesions upon necropsy along with the inflammation of the Bursa of Fabricius and haemorrhages on breast and thigh muscles. In group G-IV vaccinated with the commercial vaccine, mild enlargement of the Bursa of Fabricius was observed, while in groups G-II and G-III (Icx), the Bursa of Fabricius was found to be normal. No significant differences in body weight were observed in all the vaccinated groups.
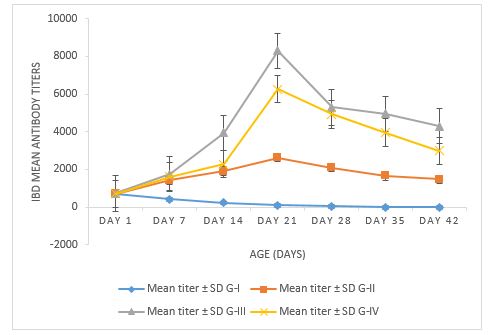
Figure 3. The comparative immune response of IBDV immune complex antigen and commercial IBDV live vaccine
Table 1. Response of Semi-SPF chicks vaccinated with Icx and commercial IBD vaccine
| Group | Vaccine | Bursa to body weight ratio post challenge (day 35) |
| GI | Control | 0.30 ± 0.89 |
| G-I | Challenge Control | 3.84 ± 0.89 |
| G-II | Icx | 0.45 ± 0.84 |
| G-III | Icx booster | 0.92 ± 0.53 |
| G-IV | Commercial | 1.07 ± 0.45 |
Table 2. Immune Response of immune complex vaccine in the natural host determined through I-ELISA
| Sera collected at | IBD Antibody Titer Level | |||||||||||
| Range (G-I) | Range (G-II) | Range (G-III) | Range (G-IV) | |||||||||
| Min. | Max. | MT ± SD | Min. | Max. | MT ± SD | Min. | Max. | MT ± SD | Min. | Max. | MT ± SD | |
| Day 1 | 540 | 850 | 719.21± 15.36 | 530 | 745 | 715.18± 12.37 | 612 | 988 | 730.21± 12.96 | 484 | 921 | 701.19± 12.63 |
| Day 7 | 260 | 564 | 407.69± 17.48 | 921 | 1752 | 1440.63± 12.84 | 1256 | 2045 | 1757.22± 12.78 | 1120 | 1918 | 1619.51± 16.80 |
| Day 14 | 154 | 558 | 225.36± 16.26 | 1263 | 2596 | 1910.24± 24.20 | 3064 | 4690 | 3950.45± 28.50 | 2163 | 2498 | 2289.79± 18.26 |
| Day 21 | 90 | 142 | 103.15± 12.22 | 2496 | 3059 | 2632.12± 15.25 | 8132 | 8365 | 8289.56± 15.53 | 5088 | 6436 | 6258.25± 16.96 |
| Day 28 | 16 | 125 | 54.25± 0.28 | 1966 | 2255 | 2103.45± 22.28 | 5124 | 5469 | 5291.14± 24.95 | 4498 | 5087 | 4921.56± 17.48 |
| Day 35 | 0 | 13 | 1.03± 0.81 | 1379 | 2078 | 1665.25± 10.47 | 4742 | 5112 | 4956.74± 12.28 | 3780 | 4132 | 3950.45± 28.50 |
| Day 42 | 0 | 0 | 0 | 1125 | 1618 | 1476.66± 15.98 | 4120 | 4460 | 4294.14± 24.95 | 2678 | 3265 | 2989.11± 10.47 |
Min = Minimum titer, Max = Maximum titer, MT = Mean titer, SD = Standard deviation
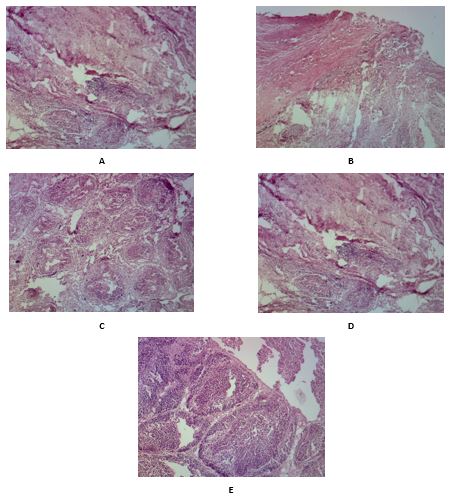
Figure 4. Bursa histopathology in different groups at 28 day-olds. A: Bursaplex vaccinated nonchallenged group showing mucosal folds, a layer of undifferentiated epithelial cells occupied the periphery of the medulla. Both cortical and medullary cellular contents are moderately reactive with closely backed small and large lymphocytes. B: Commercially vaccinated nonchallenged group showing large-sized follicles with the cortical and medullary proliferation. The medullary centers appear slightly pale and crowded by large proliferating B-cells. C: Immune complex vaccinated challenged group showed lymphoproliferative follicular tissue, highly activated cortical lymphocytes with deep basophilic nuclei, and scanty cytoplasm. D: Commercial vaccinated challenged group showing multiple immune-reactive lymphoid follicles and mild thickening of the interstitial connective tissue by fibroblastic proliferation. E: challenge control group showing massive medullary necrosis, apoptosis, heterophilic infiltration with central aggregates of necrotic and apoptotic debris, the cortical layer appeared completely necrotic with the complete lymphoid cell depletion.
The statistical analysis of the Icx and commercial vaccine treatment represents a significant P-value of ≤ 0.05. However, Table 3 represents the sudden decline in antibody titers of G-IV (commercial vaccine) birds
after the challenge compared to the G-III (Icx antigen). Icx proves to provide better and long-term immunity for poultry birds.
Table 3. Factorial analysis under CRD according to the comparative immune response of Icx and commercial vaccine
| S. O. V | DF | SS | MS | F | P-value |
| Groups | 3 | 1116.9 | 372.3 | 38.3 | 0.0 |
| Weeks | 6 | 1317.8 | 219.6 | 22.6 | 0.0 |
| Treatment | 18 | 234.5 | 13.0 | 1.3* | 0.02 |
| Error | 56 | 543.7 | 9.7 | ||
| Total | 83 | 3213.03 |
*= Significant
Although the infectious bursal disease virus was identified over 40 years ago, it continues to be a significant threat to commercial poultry worldwide. Using conventional inactivated and live infectious bursal disease virus vaccines along with strict biosecurity to control IBD had been a success story until the early 1980s until the emergence of very virulent infectious bursal disease virus strains that made the task of controlling IBD by vaccination even more challenging. Furthermore, the interference of maternally-derived antibodies with the vaccine is a major problem in early vaccination against infectious bursal disease virus with a live vaccine in birds (4, 14). To cope with this problem and for early and better vaccination, a vaccine technology is based on presenting an antigen in the context of its antibody; this antigen-antibody complex (immune complex) has been successfully applied against infectious bursal disease virus. The antigen is a whole live infectious bursal disease virus mixed with chicken egg yolk immunoglobulin (IgY) to generate an immune response in poultry birds (15, 16).
The present study was performed to explore the potential of immune complex antigen as a substitute for viral antigen against infectious bursal disease. The layer birds were used to generate antibodies expected with few immunological side effects. Using chicken IgY antibodies has several advantages over hyperimmune sera, as they reduce the painful procedure of blood collection from the animals. In terms of number, the utilization of animals is reduced because more IgY can be produced from the egg yolk. IgY antibodies were separated from the egg yolk of layer birds with ammonium sulfate (AS) precipitation. The present study results were correlated with Naveed et al. (17), who demonstrated that the AS method is superior to cation exchange chromatography in terms of purity and yield. IgY has a protective effect on animal models and can be easily used for experimentation.
This study compared three vaccination schedules against indigenous strains of infectious bursal disease virus; a commercial live attenuated IBD vaccine versus an immune complex vaccine for six weeks. The performance, Bursa to body weight ratio, Bursa histopathology and antibody immune response in vaccinated nonchallenged and vaccinated challenged birds were evaluated. All the vaccinated groups were protected against the infectious bursal disease virus challenged compared to the 60% mortality in the challenge control group. The immune complex and commercial vaccinated groups showed higher Bursa to bodyweight ratios than the control group. However, on the 28th day of age, the BB ratio in the immune complex vaccinated challenged group was significantly lower compared to the commercial vaccinated group.
Serologically, In the G-I unvaccinated control, the minimum antibody titer was 13 and the highest 850, which indicated the maternal-derived antibodies. Whereas the mean titer was highest on day 1st i.e., 719.21 ± 15.36, which declined to 0 on day 42. In G-II, the minimum antibody titer was 530, and the maximum was 3059, whereas the mean titer of 2632.12 ± 15.25 was highest on day 21 post-immunization and declined to 1476.66 ± 15.98 on day 42. In G-III, the booster dose of the Icx group showed a minimum titer of 612 and a maximum of 8365, with the highest titer indicated on day 21 post-immunization with 8289.56 ± 15.53, and it declined to 4294.14 ± 24.95 on day 42. In G-IV, the commercial vaccine revealed a minimum titer of 448 and a maximum of 6436, whereas the mean antibody titer was maximum at day 21 post-immunization with 6258.25 ± 16.96, which declined to 2989.11 ± 10.47 on day 42. The antibody titers detected through I-ELISA in the vaccinated nonchallenged groups indicated that the immune complex vaccinated group titers were significantly higher.
Similarly, after the challenge, the commercially vaccinated group (G-IV) had significantly higher antibody titers as immune complex (G-II) without a booster dose on day 28th. Still, the immune complex booster group (G-III) showed higher antibody titers and better immune response on days 28th and 35th compared to the commercial vaccine group. The IBD immune complex vaccinated birds developed mild histopathology of the Bursa of Fabricius comparable to the histopathology of commercial live attenuated vaccinated birds. The final results of the comparative mean titer were performed using factorial analysis, and the statistical analysis is correlated with the previous studies used to analyze the data through factorial analysis (17), for a comparative analysis of multiple means for the effect of three parameters IgY from egg yolk, serum, and the control.
The resulting new concept of immune complex antigen ensures the maintenance of maximum potency of the vaccine and consistent results in the field while avoiding the risk of immunosuppression. One of the strategic points in the fight against Gumboro disease is to ensure complete protection of the birds during the susceptible period, which generally lasts from 2 to 6 weeks of age. During the first 2 -3 weeks of the chick's life, protection should be obtained through the passive immunity given by the maternally derived antibodies (MDAs), as no vaccination program in the chicks will successfully cover this period. From 3 weeks of age onwards, protection should be provided by direct vaccination of the chick (active immunity). This period between the decline in passive immunity and the onset of active immunity must be as short as possible to avoid the feared immunity/defense gap (18). The immune complex antigen can provide the so-called "intelligent vaccination against IBDV" by adapting the onset of immunity to the protective needs of each individual chick, thereby decreasing the possibility of the immunity gap that can occur when other types of vaccine are used. To achieve this effect, the vaccine virus must start multiplying as soon as the MDAs are getting low. Icx, with its innovative formula, guarantees the maximum vaccine potency even in the presence of high MDAs and leads to an earlier onset of protection compared with alternative vaccines against IBDV (recombinant, live-attenuated).
Previous experimental studies indicated that live IBD vaccination might induce transient immunosuppression, leading to suboptimal vaccine responses and insufficient protection against other pathogens. The Icx antigen has revolutionized the formulation and control of vaccines to ensure the complete neutralization of live attenuated virus at the time of inoculation. This complete neutralization avoids the possibility of too early replication of the vaccine virus in a developing Bursa, leading to the feared immunosuppressive effect.
In the present study, layer birds also served as an alternative to the animals to synthesize antibody-based vaccines to reduce the painful procedure of blood collection from animals. Moreover, the ethical constraints in using animals for experimentation may be resolved. The overall study described the role of the immune complex vaccine as a potential alternative to live and recombinant infectious bursal disease virus vaccines. Icx provoked better protective immunity and protected chickens against the IBD virus challenge and may be considered a substitute for the IBDV vaccine.
Concludingly, it was evident from the results that immune complex antigen was an equally better option to enhance the antibody titer against infectious bursal disease and improve bird protection. Therefore, the immune complex antigen may also be promoted as an equally best vaccine candidate to protect poultry birds against infectious bursal disease virus.
We acknowledge Dr. Rai Shafqat, Deputy Director, CVH Faisalabad, for his kind cooperation and Dr. Mudasser Habib, Principal Scientific Officer, NIAB, Dr. Salahuddin Shah, Principal Scientific Officer, NIAB and Dr. Shahid Mahmood, Associate Professor, Microbiology, UAF, for their support and cooperation during the research.
Sanaullah Sajid wrote and performed the experimental studies, Sajjad ur Rahman supervised the study, Mashkoor Mohsin Gilani Evaluated the experimental procedures and Zia ud Din Sindhu performed the data analysis.
Conflicts of Interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Received: 2021/09/24 | Accepted: 2022/07/7 | ePublished: 2022/09/9
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








