BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1440-en.html
2- Department of Microbiology, Faculty of Advanced Sciences, Islamic Azad University of Medical Sciences, Tehran, Iran ,
Infectious diseases are one of the major causes of both morbidity and mortality rates, especially in developing countries. According to the WHO, four of the ten leading reasons for death are related to infectious diseases (1). Nowadays, gram-positive cocci, such as S. aureus, S. saprophyticus, S. epidermidis, E. faecium, and, E. faecalis are among the most prevalent bacteria causing nosocomial, skin, and systemic infections such as osteomyelitis, endocarditis, sepsis, and pneumonia. All the infections resulting from the mentioned bacteria account for more than 50% of deaths due to nosocomial infections (2). The challenges in treating infections have increased due to drug resistance.
In recent years, bacterial resistance to diverse local antibiotics, including bacitracin (3), polymyxin (4), erythromycin (5), gentamycin (6), tetracycline (7), and clindamycin (8) has been reported. This phenomenon is mainly attributed to the non-scientific, irresponsible administration, and over-the-counter availability of antibiotics for medical purposes. In fact, drug resistance occurs when bacteria change in a way that decreases or eliminates the effectiveness of drugs previously used to cure the infections (9). To overcome antibiotic resistance, it is essential to understand the mechanism of the resistance procedure. The main antibiotic resistance mechanisms entail the egress of antibiotics from the bacterial cell by efflux pumps, enzymatic destructions or alterations of antibiotic molecules, and changes in an antibiotic target that inhibits antibiotic adhesion leading to diminished activity of the medication (10). The diversity in resistance mechanisms suggests that a variety of methods are needed for defeating antibiotic resistance. These techniques include applying the synergistic activity of antibiotics and other medications, inhibiting the resistance enzymes that destroy, alter, or inactivate antibiotics, blocking the emission of antibiotics from cells or increasing the entrance of antibiotics to cells, and changing antibiotic-resistant cells physiologically (11).
One of the promising methods to overcome bacterial resistance is using metal nanoparticles in combination with antibiotics (12). Metal nanoparticles can pass through cellular impermeable membranes due to their small size and purposive design. The high surface-to-volume ratio of metal nanoparticles enables them to have the highest effective interaction with cellular membranes and walls of different pathogens (13). Moreover, these nanoparticles have been utilized to deliver antimicrobial agents to infection sites (14), improve their therapeutic index, and reduce their administration frequency (15). Antibiotic molecules can be combined with metal nanoparticles through non-covalent interactions or covalent bonds. According to the literature, both mentioned methods elevate the efficacy of antibiotics against bacteria and reduce the minimum inhibitory concentration (MIC), compared to the single antibiotic therapy (16). Currently, silver, zinc oxide, titanium, iron, and copper nanoparticles are the most common metal nanoparticles being used in antimicrobial studies due to their high antibacterial properties (17).
Nonetheless, these nanoparticles can negatively affect mammalian cells (18,19). The accumulation of silver nanoparticles has been detected in the lungs, spleen, liver, and brain of the mice exposed to silver nanoparticles (20). It was also reported that zinc nanoparticles cause toxicity, membrane damage, and elevated oxidative stress in mammalian cells (21). As a chemically ineffective material, titanium dioxide leads to some toxic effects in the form of nanoparticles, such as DNA destruction, genetic toxicity, and pulmonary inflammation (22).
Gold nanoparticles (AuNPs) demonstrate a wide range of sizes (1 nm to 8 µm), colors (brown, orange, red, and purple), and shapes (spherical, sub-octahedral, octahedral, and decahedral); thus, they have been received significant attention in several research areas (23). Furthermore, AuNPs are inert biologically and have various physicochemical properties, which make them acceptable for different biomedical uses (24). The antibacterial activity is not among the properties of pure AuNPs. However, because of their large surface area they can be used as drug carriers for antibiotic agents (25). Moreover, there are several contradictories about the toxicity and the application of AuNPs as antibiotic delivery systems; thus, more researches need to be conducted on the toxicity and adverse effects of AuNPs (26).
Here, the positive impacts of AuNPs on gentamicin, erythromycin, clindamycin, bacitracin, and polymyxin B, as antibiotic enhancers, against S. aureus, S. saprophyticus, S. epidermidis, E. faecium, and E. faecalis are explained. We hypothesized that AuNPs improve the efficacy of the evaluated antibiotic agents and decrease their MIC.
Materials
The colloidal gold nanoparticles were purchased from Nano Pad Sharif (Tehran, Iran). This colloidal solution contains gold nanoparticles homogeneously suspended in deionized water with an index of 183 dispersion. The concentration of this nano-colloid was adjusted to 100-200 µg/mL with a purity of 99%, containing reddish spherical particles with 16 nm in diameter and average zeta potential of -54.4 mV. All other chemicals were provided and applied as the chemical grade.
Bacterial Strains
The standard spp. S. aureus (ATCC 25923), S. saprophyticus (ATCC 1440), S. epidermidis (ATCC 1446), E. faecium (ATCC 29212), and E. faecalis (ATCC 1237) were prepared from the Microbial Bank of the Pharmacy Faculty of the Islamic Azad University of Medical Sciences, Tehran, Iran. All the organisms were maintained on nutrient agar plates and revived by culturing in fresh media.
Preparation of an AuNPs-antibiotic Mixture
The stock solution of each gentamycin, erythromycin, clindamycin, bacitracin, and polymyxin B was prepared by dissolving the antibiotic powder (Sigma-Aldrich) in an appropriate solvent and then vortexed. Hence, all the antibiotics go into the solution. In order to prepare the mixtures, 25 μL, 50 μL, and 75 μL of colloidal AuNP were mixed with 75 μL, 50 μL, and 25 μL of each antibiotic, respectively. Moreover, 100 μL of each antibiotic alone and 100 μL of AuNP alone were also used as the treatments. The ratios of the treatment mixtures were considered as 25:75, 50:50, 75:25, 0:100, and 100:0.
Kirby-Bauer Susceptibility Test
The standard Kirby-Bauer method was used to examine the antimicrobial properties of each mixture (27). The strains were grown on the specific media at 37°C by streak-plate procedure (27). In detail, 15 mL of the broth Muller-Hinton agar (Merck, Germany) was poured into every petri dish. After the medium had solidified, the bacteria suspension (equivalent to 0.5 McFarland) was spread over the surface of each prepared petri dish by a loop near the Bunsen burner's flame, then incubated at 37°C overnight. After that, the blank disks made from filter paper were dipped into the standard solutions of diverse mixtures of each antibiotic and AuNPs, placed on the Petri dishes, then left to incubate at 37°C overnight. Finally, the diameter of the zone of inhibition for each treatment was measured using a caliper. This method was also used to determine the zone of inhibition of each antibiotic and the AuNPs individually. All analyses were carried out in triplicate.
Determination of the Minimum Inhibitory Concentration (MIC)
The MIC refers to the lowest concentration of an antimicrobial drug that can inhibit the visible growth of a microorganism (28). This technique was utilized in cases with zones of inhibition larger than 15 mm in diameter to confirm the results of microbial assays. In order to determine MIC via broth microdilution assay based on CLSI 2017 (29), firstly, the microbial suspensions were adjusted to the turbidity of 0.5 McFarland. Next, in 96-wells microplates, a proper amount of broth medium was added to the wells and mixed with serially diluted antibiotic agents in 96-well microplates. Finally, the bacteria suspension was added. Two final wells were considered as the negative and positive control groups. Afterward, the prepared plates were incubated at 37°C overnight. In the end, the microplates were placed in a shaking incubator (model KMC65) at 150 rpm, and 37°C for 24 h. The absorbance of each well was then determined at 570 nm using a Multiskan Plus plate reader (lab systems). All experiments were performed in triplicate in order to confirm the validity of the results.
Statistical Analysis
The means of diameters of zone of inhibition for each group were compared by Duncan's Multiple Range Test (DMRT) at the probability level of 5% using the SPSS version 17.0. (SPSS Inc., Chicago, IL., USA). Moreover, the Figures were drawn utilizing Excel software (version 2016). The differences between every two groups were compared via t-test using GraphPad Prism, version 5 (San Diego, CA). The P-value less than 0.05 (P≤0.05) is considered as statistically significant different.
Figure 1 demonstrates the diameter of the zones of inhibition of each bacteria growth in the presence of free antibiotics and free-AuNPs. Moreover, different ratios of antibiotic-AuNPs were also used to evaluate their antibacterial effects against the bacteria.
Figure 1. The impact of AuNPs and antibiotics alone on the diameter of the zone of bacterial growth inhibition.
Effect of Different Ratios of Gentamicin and AuNPs
The means of diameters of zone of inhibition for this group were analysed by Duncan's Test (Table 1). Furthermore, the impact of different ratios of gentamicin and AuNPs on the diameter of the inhibition zone of the bacterial growth was shown in Figure 2. The results revealed that the smallest and the largest zones of inhibition of S. aureus, A. epidermidis, and E. faecalis growth related to AuNPs alone and the ratio of 25:75, respectively, meaning that this mixture augmented the size of the zone of inhibition at 31%, 37%, and 35% for S. aureus (21 mm), S. epidermidis (28 mm), and E. faecalis (21.6 mm), compared to gentamicin alone, respectively. Nonetheless, an elevation in the concentration of AuNPs to more than 25% led to a significant decrease in the inhibition zone of the mentioned bacteria's growth. Moreover, the simultaneous usage of gentamicin and AuNPs at all the applied ratios resulted in a significantly smaller zone of bacterial-growth inhibition for S. saprophyticus and E. faecium compared to gentamicin alone (P≤0.001 and P<0.0001, respectively). For example, the ratio of 25:75 caused an 80% decline in the efficiency of this antibiotic against E. faecium.
The MIC test reported the minimum inhibitory concentration of pure gentamycin, which was 5 µg/mL (0.01 dilution), 500 µg/mL (1 dilution), and 5 µg/mL (0.01 dilution) against S. aureus, S. saprophyticus, and S. epidermidis, respectively. These results were similar to the ratio of 25:75. In addition, the MIC of gentamycin for E. faecalis was 50 µg/mL (0.1 dilution), while the ratio of 25:75 reduced MIC, resulting in the enhanced antibacterial influence of this antibiotic. On the other hand, the mentioned mixture increased the MIC of gentamycin against E. faecium, and weakened the antibacterial effect (Table 6).

Figure 2. The impact of different ratios of AuNPs/ Gentamicin on the diameter of the zone of bacterial growth inhibition.
Table 1. Duncan's multiple range test results for the mean diameter of the zone of bacterial-growth inhibition for AuNP/ Gentamicin mixture.
| T1 | S. aureus | S. saprophyticus | S. epidermidis | E. faecium | E. faecalis |
| AuNP 0%- Gen 100% | 16.00±1.00 b | 8.66±0.33 a | 20.66±0.88 b | 34.66±0.33 a | 16.00±1.52 b |
| AuNP 25%- Gen75% | 21.00±1.15 a | 7.00±0.00 b | 28.33±0.33 a | 7.00±0.00 b | 21.66±1.66 a |
| AuNP 50%- Gen50% | 19.33±0.33 a | 7.00±0.00 b | 26.33±0.66 a | 7.00±0.00 b | 20.33±1.85 a |
| AuNP 75%- Gen 25% | 14.66±1.20 b | 7.00±0.00 b | 21.00±1.00 b | 7.00±0.00 b | 13.66±0.88 b |
| AuNP 100%- Gen0% | 7.00±0.00 c | 7.00±0.00 b | 7.00±0.00 c | 7.00±0.00 b | 7.00±0.00 c |
*Different letters (a,b,c) indicate statistical differences of Duncan's multiple range test among the groups.
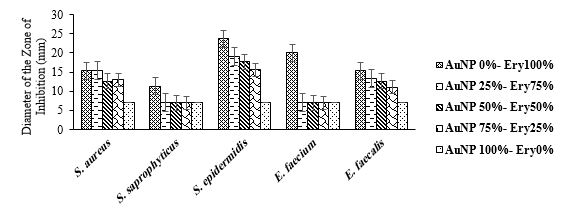
Effect of different ratios of erythromycin and AuNPs
The means of diameters of zone of inhibition for this group were analysed by Duncan's Test (Table 2). Furthermore, the impact of various ratios of erythromycin and AuNPs on the size of the inhibition zone of the bacterial growth was depicted in Figure 3. Our findings demonstrated that the zone of inhibition of S. aureus and E. faecalis growth when applying the mixture comprising 75% erythromycin and 25% AuNPs (15.3 and 13.3 mm, respectively) was approximately similar to when using erythromycin alone (15.3 and 15.3 mm, respectively). Therefore, the two groups were not significantly different in this regard (P>0.05). However, higher amounts of AuNPs led to a significantly smaller zone of inhibition against these two bacteria compared to the individual use of erythromycin. Furthermore, utilizing erythromycin and AuNPs simultaneously at all mentioned ratios reduced the diameter of the zone of inhibition for S. saprophyticus, S. epidermidis, and E. faecium compared with erythromycin alone.
The results of the MIC test indicated that the MIC of the ratio of 25:75 against S. aureus, S. epidermidis, E. faecium, and E. faecalis was higher than that of erythromycin alone. The findings suggest the reduced antibacterial influence of this antibiotic when mixed with AuNPs. Moreover, the MIC of pure erythromycin against S. saprophyticus was 750 µg/mL (1 in dilution), which was similar to the mixture of 25 μL AuNPs and 75 μL erythromycin (Table 6). Overall, it could be stated that the incorporation of AuNPs did not significantly improve the efficacy of erythromycin in inhibiting the growth of gram-positive cocci.
Figure 3. The impact of different ratios of AuNPs/ Erythromycin on the diameter of the zone of bacterial growth inhibition.
Table 2. Duncan's multiple range test results for the mean diameter of the zone of bacterial-growth inhibition for AuNP/ Erythromycin mixture.
| T2 | S. aureus | S. saprophyticus | S. epidermidis | E. faecium | E. faecalis |
| AuNP 0%- Ery 100% | 15.33±0.33 a | 11.33±1.85 a | 23.66±1.85 a | 20.00±0.57 a | 15.33±0.88 a |
| AuNP 25%- Ery75% | 15.33±0.88 a | 7.00±0.00 b | 19.00±1.52 b | 7.00±0.00 b | 13.33±0.88 ab |
| AuNP 50%- Ery 50% | 12.66±1.20 b | 7.00±0.00 b | 17.66±1.20 b | 7.00±0.00 b | 12.66±1.20 ab |
| AuNP 75%- Ery 25% | 13.00±0.57 ab | 7.00±0.00 b | 15.66±1.20 b | 7.00±0.00 b | 11.00±1.52 b |
| AuNP 100%- Ery 0% | 7.00±0.00 c | 7.00±0.00 b | 7.00±0.00 c | 7.00±0.00 b | 7.00±0.00 c |
*Different letters (a,b,c) indicate statistical differences of Duncan's multiple range test among the groups.
Effect of Different Ratios of Clindamycin and AuNPs
The means of diameters of zone of inhibition for this group were analysed by Duncan's Test (Table 3). Furthermore, the results showed that the simultaneous application of clindamycin with AuNPs positively impacted inhibiting the growth of E. faecalis. The 25:75 ratio and AuNPs alone showed the largest and smallest inhibition zones for E. faecalis, respectively. The 25:75, 50:50, and 75:25 ratios caused a 184% (P<0.0001), a 178% (P<0.0001), and a 133% (P≤0.0002) considerable increase in the size of the zone of inhibition for E. faecalis, as compared to clindamycin alone, respectively. On the other hand, the mixture of this antibiotic with AuNPs in all ratios reduced the diameter of the zone of inhibition for S. aureus, S. saprophyticus, S. epidermidis, and E. faecium, in comparison with individual clindamycin (Figure 4).
According to the MIC test, the MIC of pure clindamycin for S. aureus, S. epidermidis, and E. faecium was similar to the ratio of 25:75. However, the MIC of 25:75 ratio against E. faecalis was lower than that of clindamycin alone, indicating the improved antibacterial effect (Table 6).
Figure 4. The impact of different ratios of AuNPs/ Clindamycin on the diameter of the zone of bacterial growth inhibition.
Table 3. Duncan's multiple range test results for the mean diameter of the zone of bacterial-growth inhibition for AuNP/ Clindamycin mixture.
| T3 | S. aureus | S. saprophyticus | S. epidermidis | E. faecium | E. faecalis |
| AuNP 0%- Clin 100% | 42.00±2.08 a | 39.00±1.00 a | 39.00±0.57 a | 40.33±1.20 a | 11.00±1.00 c |
| AuNP 25%- Clin 75% | 30.33±0.33 b | 34.00±0.33 b | 33.66±0.66 b | 39.33±0.66 a | 31.33±0.33 a |
| AuNP 50%- Clin 50% | 28.00±0.57 bc | 32.33±0.33 c | 31.00±0.00 c | 36.66±0.33 b | 30.66±0.33 a |
| AuNP 75%- Clin 25% | 27.00±0.57 c | 29.33±0.33 d | 27.33±0.66 d | 35.33±0.33 b | 25.66±1.76 b |
| AuNP 100%- Clin 0% | 7.00±0.00 d | 7.00±0.00 e | 7.00±0.00 e | 7.00±0.00 c | 7.00±0.00 d |
*Different letters (a,b,c,d,e) indicate statistical differences of Duncan's multiple range test among the groups.
Effect of different ratios of bacitracin and AuNPs
The means of diameters of zone of inhibition for this group were analysed by Duncan's Test (Table 4). Furthermore, the present study's findings revealed that mixing bacitracin with AuNPs in various ratios significantly decreased the size of the zone of inhibition against gram-positive cocci (except for S. epidermidis) compared to bacitracin alone. For example, the 25:75 ratio significantly reduced the size of the zone of inhibition at 37% (P≤0.003), 32% (P≤0.001), and 36% (P ≤ 0.01) for S. saprophyticus, E. faecium, and E. faecalis, respectively. However, the mentioned mixture led to a 10% rise in the diameter of the zone of inhibition for S. epidermidis in comparison with utilizing bacitracin alone, which was not statistically significant (P>0.05) (Figure 5).
It could be concluded from the results of the MIC test that the MIC of pure bacitracin against the bacteria spp. was similar to the 25:75 ratio (Table 6).

Figure 5. The impact of different ratios of AuNPs/ Bacitracin on the diameter of the zone of bacterial growth inhibition.
Table 4. Duncan's multiple range test results for the mean diameter of the zone of bacterial-growth inhibition for AuNP/ Bacitracin mixture.
| T4 | S. aureus | S. saprophyticus | S. epidermidis | E. faecium | E. faecalis |
| AuNP 0%- Bac 100% | 9.00±0.00 a | 14.33±1.45 a | 8.16±0.60 ab | 14.33±0.66 a | 11.00±1.52 a |
| AuNP 25%- Bac 75% | 9.00±0.00 a | 9.00±0.00 b | 9.00±0.00 a | 9.66±0.66 b | 7.00±0.00 b |
| AuNP 50%- Bac 50% | 8.00±0.00 a | 8.00±0.16 b | 8.00±0.00 b | 8.66±0.66 bc | 7.66±0.33 b |
| AuNP 75%- Bac 25% | 7.00±0.00 a | 7.16±0.16 b | 7.00±0.00 c | 7.66±0.66 c | 7.00±0.00 b |
| AuNP 100%- Bac 0% | 7.00±0.00 a | 7.00±0.00 b | 7.00±0.00 c | 7.00±0.00 c | 7.00±0.00 b |
*Different letters (a,b,c) indicate statistical differences of Duncan's multiple range test among the groups.
Effect of Different Ratios of Polymyxin B and AuNPs
The means of diameters of zone of inhibition for this group were analysed by Duncan's Test (Table 5). Furthermore, the influence of diverse ratios of polymyxin B and AuNPs on the diameter of the zone of inhibition against the bacteria was demonstrated in Figure 6. According to the results, simultaneous usage of polymyxin with AuNPs with the ratio of 50:50 significantly augmented the zone of inhibition against S. saprophyticus (P<0.0001) compared with polymyxin B alone. The 50:50 ratio increased the zone of inhibition at 8% against S. saprophyticus, compared to pure polymyxin B. On the other hand, mixing this antibiotic with AuNPs in different ratios significantly led to smaller zones of inhibition for other investigated bacteria. Moreover, it should be noted that the zone of inhibition for the 25:75 and 00:100 ratios was approximately similar.
According to the results of the MIC test, the MIC of the 25:75 ratio against S. saprophyticus, E. faecium, and E. faecalis was similar to the MIC of polymyxin B alone. Nonetheless, the MIC of the 25:75 for S. aureus was lower than that of individual polymyxin B, indicating the promoted antibacterial impact of this compound (Table 6).
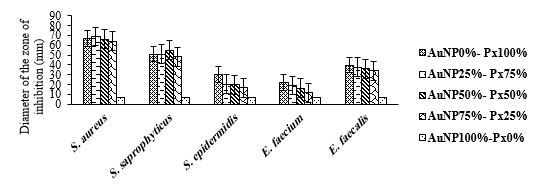
Figure 6. The impact of different ratios of AuNPs/ Polymyxin B on the diameter of the zone of bacterial growth inhibition.
Table 5. Duncan's multiple range test results for the mean diameter of the zone of bacterial-growth inhibition for AuNP/ Polymyxin B mixture.
| T4 | S. aureus | S. saprophyticus | S. epidermidis | E. faecium | E. faecalis |
| AuNP 0%- PB 100% | 67.00±0.00 ab | 50.83±0.44 b | 30.00±0.00 a | 22.33±1.76 a | 39.66±0.33 a |
| AuNP 25%- PB 75% | 68.66±0.33 a | 51.00±1.15 b | 20.33±0.33 b | 19.00±1.00 ab | 37.66±0.33 b |
| AuNP 50%- PB 50% | 66.00±0.57 b | 55.00±0.00 a | 20.00±0.00 b | 16.33±0.88 b | 36.00±0.00 c |
| AuNP 75%- PB 25% | 64.00±1.00 c | 48.33±1.33 b | 16.66±0.66 c | 11.66±1.20 c | 34.00±0.57 d |
| AuNP 100%- PB 0% | 7.00±0.00 d | 7.00±0.00 c | 7.00±0.00 d | 7.00±0.00 d | 7.00±0.00 e |
*Different letters (a,b,c,d,e) indicate statistical differences in Duncan's multiple range test among the groups.
Table 6. MIC of AuNPs mixed with different ratios of Antibiotics against Bacteria spp.
| S. aureus | S. saprophyticus | S. epidermidis | E. faecium | E. faecalis | |
| AuNP 0-Gen 100 | 0.1 dilution (50 µg/mL) |
0.0001 dilution (0.05 µg/mL) |
0.01 dilution (5 µg/mL) |
1 dilution (500 µg/mL) |
0.01 dilution (5 µg/mL) |
| AuNP 25-Gen 75 | 0.01 dilution (5 µg/mL) |
1 dilution (500 µg/mL) |
0.01 dilution (5 µg/mL) |
1 dilution (500 µg/mL) |
0.01 dilution (5 µg/mL) |
| AuNP 0-Ery 100 | 0.01 dilution (7.5 µg/mL) |
0.001 dilution (0.75 µg/mL) |
0.001 dilution (0.75 µg/mL) |
1 dilution (750 µg/mL) |
0.01 dilution (7.5 µg/mL) |
| AuNP 25-Ery 75 | 1 dilution (750 µg/mL) |
1 dilution (750 µg/mL) |
(7.5 µg/mL)
|
1 dilution (750 µg/mL) |
(75 µg/mL)
|
| AuNP 0-Clin 100 | 0.1 dilution (10 µg/mL) |
0.01 dilution (1 µg/mL) |
0.01 dilution (1 µg/mL) |
0.01 dilution (1 µg/mL) |
0.01 dilution (1 µg/mL) |
| AuNP 25-Clin 75 | 0.001 dilution (0.1 µg/mL) |
0.01 dilution (1 µg/mL) |
0.01 dilution (1 µg/mL) |
0.1 dilution (10 µg/mL) |
0.01 dilution (1 µg/mL) |
| AuNP 0-Bac 100 | 1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
| AuNP 25-Bac 75 | 1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
1 dilution (500 µg/mL) |
| AuNP 0-Px 100 | 0.01 dilution (150 µg/mL) |
0.001 dilution (15 µg/mL) |
0.01 dilution (150 µg/mL) |
0.001 dilution (15 µg/mL) |
0.01 dilution (150 µg/mL) |
| AuNP 25-Px 75 | 0.01 dilution (150 µg/mL) |
0.001 dilution (15 µg/mL) |
1 dilution (15000 µg/mL) |
0.001 dilution (15 µg/mL) |
0.001 dilution (15 µg/mL) |
Numerous antimicrobial studies have investigated AuNPs combined with local and systemic antibiotics and diverse polymers (30). The present study evaluates the antibacterial impact of gold nanoparticles mixed with gentamycin, erythromycin, clindamycin bacitracin, and polymyxin B on gram-positive cocci, including S. aureus, S. saprophyticus, S. epidermidis, E. faecium, and E. faecalis. Our findings revealed that AuNPs alone did not indicate any antibacterial effects against the tested bacterial spp., according to the results of the disk diffusion method. One similar study conducted by Grace and Pandian confirmed that AuNPs showed no antibacterial activities against various bacteria such as P. aeruginosa, S. aureus, and E. coli. In contrast, coating AuNPs with antibiotics increased their antibacterial effect (31). Another study proved this idea and demonstrated that even in the presence of a media containing high levels of ampicillin, AuNPs without binding to ampicillin showed no antibacterial effect against β-lactam resistant bacteria (32).
The current study also showed that the 25:75 ratio of AuNPs with gentamicin and the 50:50 ratio of these nanoparticles with polymyxin B led to more extensive zones of inhibition against S. aureus (P≤0.005) and S. saprophyticus (P<0.0001), in comparison with pure antibiotics, respectively. In addition, an increase in the diameter of the zone of inhibition for S. epidermidis was observed when applying 25 μL AuNPs with 75 μL gentamycin (P≤0.0001) or bacitracin (P≤0.07). Similarly, this increase was found against E. faecalis when applying the 25:75 ratio of AuNPs with gentamycin (P≤0.01) or clindamycin (P<0.0001). However, MIC did not alter when applying a 25:75 ratio of AuNPs and gentamycin against S. aureus and S. epidermidis, a 25:75 ratio of AuNPs and bacitracin against S. epidermidis, and a 50:50 ratio of AuNPs and polymyxin B against S. saprophyticus. This might be attributed to the fact that in the present study, serial dilutions for the MIC test were prepared at 0.1 ratios, rather than using two-fold serial dilution.
The results of this study were consistent with the findings of some previous studies that supported the idea that combining different antibiotics with AuNPs significantly improved the antibacterial effect of antibiotics against gram-positive and gram-negative bacteria. For instance, Payne et al. showed that conjugating kanamycin with AuNPs promoted the influence of this antibiotic. In addition, the MIC of Kan-AuNPs reduced against bacteria which were sensitive and resistant to kanamycin, when compared to free kanamycin. The mechanism of action is probably due to the accumulation of the mixture of kanamycin and AuNPs in the bacterial membrane and cytosol. The AuNPs can settle in the entrance of the bacterial membrane and elevate the local concentration of antibiotics, resulting in the leakage of cytoplasmic content and the death of bacterial cells (33).
Furthermore, Rai et al. reported that cefaclor conjugated with AuNPs imposed a higher antibacterial effect on gram-positive (e.g., S. aureus) and gram-negative (E. coli) bacteria than cefaclor alone. Cefaclor inhibits the formation of the peptidoglycan layer in bacterium leading to the generation of some pores in the bacterial cell wall. Moreover, AuNPs caused some pits to form in the bacterial cell wall resulting in the leakage of bacterial content to the outer environment and cell death (34). Zawrah et al. also concluded that the diameter of the zone of inhibition using AuNPs, ciprofloxacin, and AuNPs-coated ciprofloxacin was 12, 26, and 30 mm, respectively. In addition, the results of the MIC test revealed that combining ciprofloxacin with AuNPs led to the reduction of MIC from 0.19 to 0.097 µg/mL. These authors claimed that the enhanced antibacterial impact of ciprofloxacin in the presence of AuNPs might be attributed to the destruction of the pathogen cell wall and isolation from the cell membrane (35).
Similarly, Saha et al. demonstrated that the conjugation of AuNPs with ampicillin, streptomycin, and kanamycin augmented the size of the zone of inhibition against E. coli. Additionally, the MIC of AuNPs combined with ampicillin, streptomycin, and kanamycin reduced 10%, 50%, and 60%, respectively (36). In a study in 2019, the effect of various nanoparticles such as gold, silver, and platinum on Bacillus sp. was investigated. Nishanti et al. showed that the combination of streptomycin and these nanoparticles indicated enhanced antibacterial effect against the bacteria, suggesting the synergistic effect between these nanoparticles and streptomycin. Notably, the combination of streptomycin with AuNPs showed a 100%-fold increase in antibacterial effect.
Meanwhile, silver and platinum nanoparticles increased streptomycin activity by 87.5% and 37.5%, respectively, which might be due to the binding reaction between the antibiotic and the nanoparticles (37). Gold nanoparticles appear to be able to facilitate the entry of antibiotics into the bacterial cell or increase the accumulation of antibiotics at the site of infection. This increase in concentration may be sufficient to overcome the resistance mechanism. Efflux pumps in bacterial cells are the major cause of antibiotic resistance. It is possible that AuNPs inhibit the ability of these pumps to take antibiotics out of the bacterial cell and cause the antibiotic to remain inside the microorganism (38,39). In the foregoing investigations, contrary to the current study, the conjugation technique was used for mixing AuNPs with antibiotics. Physical adsorption of antibiotics with AuNPs can improve the efficacy and enhance the stability against heat shock and extend storage at 25ºC (40).
Conversely, Burygin et al. indicated that the mixture of gentamycin with 15-nm colloidal AuNPs did not improve the antibacterial influence of gentamycin against E. coli. They stated that to elevate the antibiotic effect, the antibiotic should first be chemically attached to the surface of AuNPs, and form stable conjugates with nanoparticles (41). This result is in line with the current study, which showed that applying colloidal AuNPs mixed with gentamycin without conjugation led to a significantly smaller zone of inhibition against some species, including S. saprophyticus and E. faecium. In spite of AuNPs, silver nanoparticles mixed with antibiotics have shown synergistic effects. In a study by Shahverdi et al., the antibacterial activities of penicillin G, amoxicillin, erythromycin, clindamycin, and vancomycin mixed with AgNPs enhanced against E. coli and S. aureus, which might be because of the binding reaction between antibiotic and AgNPs (42).
Although several studies have indicated that simple mixing of antibiotics with AuNPs cannot enhance the antibacterial activity of antibiotics and stable conjugation gives more clear results, our findings indicated that simple mixing of AuNPs with antibiotics could also increase the antibacterial effect against some gram-positive cocci. Therefore, this method might also be preferred due to its lower costs and simplicity.
However, it is suggested to perform further studies to understand the exact mechanism of AuNPs in the presence of antibiotics. Additionally, more studies on toxicity should be investigated to justify the safe use of AuNPs as drug delivery systems.
Nanotechnology provides valuable strategies to overcome antimicrobial resistance. In this study, the effect of colloidal AuNPs on the antibacterial activity of five antibiotics was evaluated. The mixture of AuNPs with some antibiotics at the ratio of 25:75 showed higher antibacterial activities against some gram-positive cocci than individual antibiotics. However, in order to get more apparent results, preparing stable conjugates of AuNPs coated with antibiotics rather than simple mixing is suggested. Moreover, more studies on the in vivo activities and toxicity of AuNPs may help researchers obtain more comprehensive conclusions.
The authors thank Islamic Azad University of Medical Sciences, Faculty of Pharmacy, for providing facilities and equipment.
None.
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2021/08/19 | Accepted: 2022/02/18 | ePublished: 2022/05/25
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |