BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1411-en.html
2- Department of Microbiology, Faculty of Basic Sciences, Shahrekord Branch, Islamic Azad University Shahrekord, Shahrekord, Iran ,
3- Department of Food Hygiene and Public Health, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
4- Cellular and Developmental Research Center, Faculty of Basic Sciences, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
Fermented dairy products are a good source for isolating microorganisms (1, 2). Cottage cheeses made from raw milk have a high genetic diversity and a more complex microbial ecosystem than other dairy products (3). Cheese traditionally produced from raw milk has a variety of genus, species, and native microbial flora of the milk used in the preparation process (4). Cheese is produced due to the coagulation of casein by the renin enzyme or similar enzymes in the presence of lactic acid produced by microorganisms (5). Lactic acid bacteria that become part of the predominant microbial flora during the cottage cheese ripening (after one month) are often from the facultatively heterofermentative Lactobacilli (FHL) (6). Lactic acid bacteria are dietary supplements known for their fermentability and health and nutritional benefits. For this reason, they have beneficial effects on the host and help balance the microbial flora of the intestine, such as Lactobacillus (7).
Lactobacilli are a type of lactic acid bacteria considered safe bacteria. These bacteria are gram-positive, non-spores, catalase-negative, and are usually immobile (8). Lactobacilli have potent antagonistic activity against many microorganisms, including food spoilage organisms and pathogens. The production of secondary metabolites, lactic acid, and the resulting decrease in pH are the main factors in food preservation. In addition, some strains can help preserve fermented foods producing other inhibitors, such as bacteriocins (9). Lactobacilli produce various compounds such as Hydrogen peroxide and organic acids during lactic fermentation that can have inhibitory effects on many microorganisms (10). Due to increasing antibiotic resistance and side effects of chemical drugs, using alternative therapies is essential (11). These bacteria and their metabolites can be used therapeutically (12). Studies confirm the positive role of lactic acid bacteria in inhibiting pathogens (13, 14).
Due to its long-term use in various food products that are traditionally fermented, Lactobacillus brevis, as an essential member of the genus Lactobacillus, has a GRAS (generally recognized as safe) status that is isolated from milk, cheese, mouth, and gastrointestinal tract (15). The aim of this study was to isolate L. brevis from cottage cheese of Bazoft, Chaharmahal and Bakhtiari Province, Iran, and investigate its antibiotic resistance and antimicrobial ability of supernatant (culture supernatant) on pathogenic bacterial strains.
Sampling
In this study, 10 samples of cottage cheese were randomly collected from rural areas of Bazoft, Chaharmahal and Bakhtiari Province, Iran. The samples were transferred to the microbiology laboratory of Islamic Azad University Shahrekord Branch under cold conditions and kept at 4°C until the beginning of the experiment.
Sample Preparation and Initial Culture
Serial dilution of samples in sterile physiological solution was obtained at 10-6 (16). Then, for initial isolation, 1 ml of each cottage cheese sample was added to 9 mL of MRS broth (Merck, Germany) (de MAN, ROGOSA, and SHARPE). The samples were placed in an anaerobic jar for 24-48 hours at 37°C. Then, to obtain a single colony from the samples, the streak plate method was performed using a sterile inoculation needle on MRS agar and incubated for 37-48 hours at 37°C (17).
Morphological Study
First, Gram's method was performed for each colony, and then they were examined and recorded using an optical microscope at 100x magnification (18).
Biochemical Properties Investigation
Oxidase, catalase, motion, indole, endospore, and H2S tests were used to identify Lactobacillus. Also, the ability to grow at 15 and 45°C after 48 hours was evaluated (19, 20).
Carbohydrate's Fermentation
A Carbohydrate-free MRS medium was used for this purpose. Nine mL of MRS broth medium containing one percent of the desired carbohydrates (Arabinose, cellobiose, Melezitose, Melibiose, Raffinose, Ribose, and Sucrose) and phenol-red reagent were added to each test tube. Then one mL of the desired bacteria (107 CFU/mL) was inoculated into each tube and incubated for 48 hours at 37°C. Turning the red color of the environment to yellow means consuming the desired sugar. The results of carbohydrate fermentation were compared with the standard table of Bergey's Manual of Systematic Bacteriology after the experiment (21).
Molecular Identification and Sequencing
Polymerase chain reaction (PCR) was used to identify and confirm the bacteria by molecular method. For this purpose, DNA was first extracted from the bacterial sample according to the protocol of the DNA extraction kit (Sinaclon, Iran). The extracted sample was used as a template for the PCR reaction. Sequence (5'-CTCAAAACTAAACAAAGTTTC-3') as forward primer and sequence (5'-CTTGTACACACC GCCCGTCA-3') as reverse primer were used (22). PCR reaction in 25 μL volume including 12.5 μL PCR buffer with 10 times density, 25 μM forward and reversed primer 0.5 μL each, 1 μL of 50 mM Magnesium chloride, 4 μL of 1.25 mM of dNTPs, 2.5 units of Taq DNA Polymerase enzyme, and 4 μL of extracted DNA, was performed. Reaction in a thermal cycler (FlexCycler2, Germany) with temperature conditions of 5 minutes, denaturation at 95°C and then 30 cycles including denaturation at 95°C for 1 minute, annealing at 55°C for 1 minute, extension at 72°C for 1 minute and final extension at 72°C for 10 minutes was performed. The product was electrophoresed in 1% agarose gel and then examined on gel doc (AzmaCell, Iran). Finally, the PCR product was sent to Sinaclon, Iran, for sequencing.
Evaluation of Antimicrobial Properties of Lactobacillus brevis
Preparation of Supernatant
Purified Lactobacillus brevis was cultured on MRS broth and was incubated under anaerobic conditions at 37°C to obtain turbidity of 0.5 McFarland. Then, to prepare the supernatant, the selected culture of Lactobacillus brevis was centrifuged at 4000 rpm for 4 minutes, and then the supernatant was filtered using a 0.22-micron filter (23).
Preparation of Pathogenic Bacteria
Five strains of pathogenic bacteria, including Escherichia coli (ATCC 2592), Staphylococcus aureus (ATCC 25923), Salmonella typhimurium (ATCC 14028), Pseudomonas aeruginosa (ATCC 27853), and Enterococcus faecalis (ATCC 29212), were obtained from Pasteur Institute of Iran and cultured in Nutrient broth. Then they were used with turbidity equivalent to 0.5 McFarland (24).
Antimicrobial Activity
Mueller-Hinton agar (MHA) (Merck, Germany) medium was used to evaluate the antimicrobial activity of L. brevis. For this purpose, two agar well and disk diffusion methods were used. In both methods, the MRS Agar plate was a positive control for L. brevis growth (25, 26).
Disk Diffusion Method
The 6 mm diameter paper disks (Padtan Teb, Iran) were soaked in Lactobacillus brevis supernatant for 5 minutes, then placed at 37°C to dry completely. Selected pathogenic bacteria cultured in Nutrient broth (Liofilchem, Italy) McFarland 0.5 were cultured on Mueller-Hinton agar plate using a swab. The disk impregnated with L. brevis supernatant was then placed on the surface of the Mueller-Hinton agar medium. After 24 hours of incubation at 37°C, the inhibitory zone diameter was measured (25).
Agar Well Diffusion Method
During this method, the suspension of pathogenic bacteria in nutrient broth medium (0.5 McFarland) was cultured on Mueller-Hinton agar medium with a sterile swab. Then, wells were made on the medium using a sterile Pasteur pipette, and 30 μL of L. brevis supernatant was poured into the wells. After drying, the plates were incubated in a 37°C incubator for 24 hours. Then inhibition zone diameter created by L. brevis against each pathogenic bacterium was measured (26).
Antibiotic Resistance
In this study, antibiotics Vancomycin (30 µg), Kanamycin (30 µg), Gentamicin (10 µg), Nalidixic acid (30 µg), Ampicillin (10 µg), Erythromycin (25 µg), Tetracycline (30 µg) (Padtan Teb, Iran) were used. To evaluate the resistance of L. brevis to the mentioned antibiotics, first, the desired strain with a density of 0.5 McFarland was cultured on MRS Agar medium. Then antibiotic discs with a specific density at a distance of 4 cm were placed on the plate surface. The plate was then incubated for 24 hours at 37°C. After this period, the inhibition zone diameter around the disc was measured, resistance and sensitivity to the antibiotic were evaluated. Also, MRS Agar medium was used to culture the positive control strain (27, 28).
A total of thirteen microorganisms were isolated, including gram-negative and gram-positive bacilli and cocci. Of these thirteen microorganisms, eight isolates were gram-positive bacilli. Six isolates tested negative for oxidase, catalase, indole, motility, and spore tests in this group and were considered as possible lactobacilli (Table 1).
Table 1. Morphological and biochemical characteristics of bacteria isolated from cottage cheese
| Isolate | Morphology | Gram | Oxidase | Catalase | Indole | Motility | Spore |
|---|---|---|---|---|---|---|---|
| PA1 | Bacilli | positive | - | - | - | - | - |
| PA2 | Bacilli | negative | + | + | - | + | - |
| PA3 | Bacilli | positive | - | - | - | - | - |
| PA4 | Cocci | positive | - | - | - | + | + |
| PA5 | Bacilli | positive | + | + | - | - | + |
| PA6 | Bacilli | positive | - | - | - | - | - |
| PA7 | Cocci | positive | - | + | + | - | - |
| PA8 | Bacilli | positive | + | + | - | - | - |
| PA9 | Cocci | positive | + | - | - | + | - |
| PA10 | Bacilli | positive | - | - | - | - | - |
| PA11 | Bacilli | negative | + | - | + | - | - |
| PA12 | Bacilli | positive | - | - | - | - | - |
| PA13 | Bacilli | positive | - | - | - | - | - |
Sugar Consumption by Bacteria
Carbohydrate fermentation experiments, the results of bacterial sugar consumption, and the ability to grow at temperatures 15 and 45°C are expressed in Table 2.
Based on the results of biochemical tests (Tables 1 and 2), the PA6 isolate was selected as L. brevis according to Bergey's Manual of Systematic Bacteriology.
Confirmation of Identification using PCR and Sequencing
To confirm the genus Lactobacillus, polymerase chain reaction (PCR) was used for the 16 s rRNA gene. The isolates were examined, and the formation of a 195 bp band by electrophoresis was confirmed by the biochemical diagnosis (Figure 1).
Table 2. Fermentation of carbohydrate test results
| Isolate | Arabinose | Cellobiose | Melezitose | Melibiose | Raffinose | Ribose | Sucrose | Growth temperature 15/45 |
| PA1 | - | + | + | - | - | + | + | +/- |
| PA3 | + | + | - | + | + | + | + | -/+ |
| PA6 | - | + | + | - | - | + | + | +/- |
| PA10 | + | - | + | + | + | + | + | -/+ |
| PA12 | - | + | + | - | - | + | + | +/- |
| PA13 | + | + | - | + | + | + | + | +/- |

Figure 1. PCR test results. The six isolates had a band of 195 bp, which was confirmed as Lactobacillus. 1) 100bp Ladder 2-7) isolates with 195 bp band as Lactobacillus 8) non-Lactobacillus with 350 bp band 9) Positive Control (Lactobacillus casei PTCC 1608) 10) negative control
Sequencing
PCR product of isolate PA6 was sent to Sinaclon for sequencing. The results of PCR product sequencing after comparing the sequences with the data in NCBI and BLAST analysis of the obtained sequence showed the similarity of PA6 isolate with L. brevis, which showed the confirmation of biochemical results and correct species detection (Figure 2).
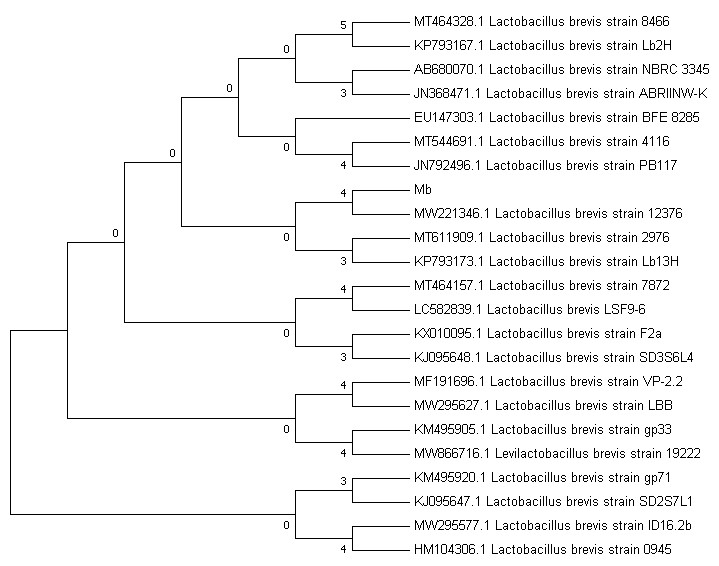
Figure 2. Phylogenetic tree of the selected Lactobacillus brevis
Antibiotic Resistance
The results of the antibiotic resistance test are demonstrated in Table 3. L. brevis was susceptible to Vancomycin and Gentamicin; this strain also was intermediate susceptible to Nalidixic acid and Kana-mycin and was susceptible to Erythromycin and Kana-mycin, Tetracycline, Ampicillin, and Chloramphenicol.
Table 3. Resistance of Lactobacillus brevis isolates against some common antibiotics
| Strain | Erythromycin | Kanamycin | Tetracycline | Nalidixic acid | Gentamicin | Ampicillin | Chloramphenicol | Vancomycin |
| Lactobacillus brevis | S | I | S | I | R | S | S | R |
Investigation of Antimicrobial Activity
The antimicrobial activity of L. brevis supernatant against five pathogenic strains was investigated by the disk and well diffusion methods (Figures 3 and 4). The results showed that the inhibitory ability varied from 9 to 19 mm. In both methods, the highest inhibitory effect was on the bacterium Salmonella typhimurium, a gram-negative bacterium and the cause of one of the most common food poisonings, and the least inhibitory effect was on Enterococcus faecalis, which is a gram-positive facultative anaerobic bacterium.
The results of the well diffusion method in (Figure 3) show the favorable inhibitory effect of L. brevis. During this method, the highest inhibitory effect was against S. typhimurium with inhibition of 19 mm, and the lowest inhibitory effect against E. faecalis with inhibition of 11 mm.
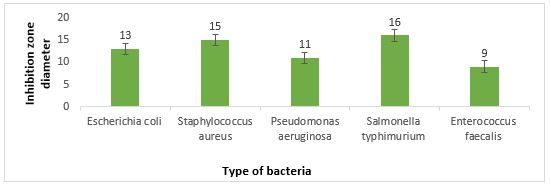
The results obtained in the disk diffusion method (Figure 4) show the inhibitory effect of L. brevis. During this method, the highest inhibitory effect was against S. typhimurium with inhibition of 16 mm, and the lowest inhibitory effect was against E. faecalis with inhibition of 9 mm.
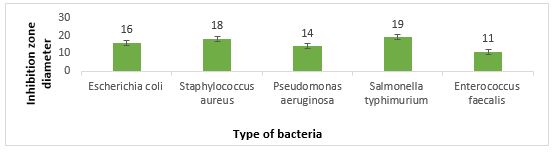
Figure 3. Inhibition zone diameter against pathogenic bacteria using well diffusion method
Figure 4. Inhibition zone diameter against pathogenic bacteria by disk diffusion method
The diversity of Lactobacillus in food products and different geographical conditions is so great that this diversity in dairy products in the world is complex and widespread. For this reason, more research should be done to achieve suitable strains with specific functional characteristics (29-31). Cheese is one of the dairy products widely available in hundreds of different types (32). The cheese made in Bazoft is one of the types of cheese that has been little studied. Therefore, it is a good source for isolating microbial strains. MRS medium has been used to isolate lactobacilli in different sources. Identification of lactic acid bacteria is based on biochemical, morphological, and molecular properties (33, 34).
The first part of this study was about the morphological and biochemical identification of L. brevis and its isolation from cottage cheese. Then, the molecular method was used to confirm biochemical tests (22). According to our findings, the rod-shaped bacteria with diverse cell arrangements, gram-positive, catalase-negative, and non-spores, belong to the genus Lactobacillus from the lactic acid family. In this study, all isolates could not produce gas from glucose, so they were placed in the category of Facultatively Heterofermentative Lactobacilli. Isolates that grew at 15°C and could not grow at 45°C are also classified as mesophilic microorganisms (19, 20). According to Bergey's Manual of Systematic Bacteriology, L. brevis can ferment the sugars Arabinose, Maltose, Ribose, Melibiose, and Sucrose but can also not ferment Mannose Melezitose (21). Based on this, L. brevis was isolated from the cottage cheese and then confirmed by the molecular method. In this study, six bacteria out of 10 isolates from cottage cheese were identified as Lactobacillus, including two isolates of Lactobacillus plantarum, two isolates of Lactobacillus casei, one isolate of Lactobacillus fermentum, and one isolate of L. brevis.
In the study of Hejazi et al. in 2012, 8 out of 22 isolates were identified as Lactobacillus, which included five species of Lactobacillus plantarum, two species of Lactobacillus brevis, and one species of Lactobacillus casei (35). In 2014, Singh and Singh isolated several Lactobacillus species, including L. plantarum, Lactobacillus paracasei, Lactobacillus rhamnosus, Lactobacillus curvatus, and L. brevis, from ripped cheddar cheese (36).
In 2019, Abdali et al. identified 10 isolates from traditional Iranian cheese as Lactobacillus, of which six species were L. plantarum and the others were Lactobacillus paracasei, Lactobacillus acidophilus, L. brevis, and Lactobacillus buchneri (37). In 2020, Zhang et al. isolated a total of 18 bacteria as Lactobacillus from cheese, including 5 isolates of L. plantarum, four isolates of L. brevis, two isolates of Lactobacillus fermentum, three isolates of L. rhamnosus, three isolates of Lactobacillus furfuricola, and one isolate of L. paracasei (38).
The second part of this study was about the antimicrobial properties of L. brevis. Searching for safe, natural, and accessible antimicrobials has always been of interest to researchers. On the other hand, Lactobacillus bacteria are GRAS due to their antimi-crobial properties and non-pathogenicity and can be an excellent alternative to use as antimicrobials (39-41). Lactobacilli form a significant part of the natural intestinal flora of animals and humans (42). They produce various antimicrobials such as Organic acids, Diacetyl, Acetone, Hydrogen peroxide, Retr-ocyclin, Antifungal peptides, and Bacteriocin. Inhib-ition of pa-thogenic activity by these bacteria can play an esse-ntial role in the health of individuals by combating infections caused by common pathogens of the gastrointestinal tract (43). It also prevents the growth of spoilage and food poisoning microorg-anisms, incr-easing the durability and the safety of food products (44). In this study, the well diffusion method had better and more positive results than the disk diffu-sion method, and L. brevis showed better inhibitory effects in the well diffusion method. L. brevis supernatant showed the highest inhibitory effect by well diffusion method against Salmonella Typhimu-rium with a growth inhibition zone of 19 mm and the least inhibitory effect by disk diffusion method with a growth inhibition zone of 9 mm against E. faecalis.
In a 2017 study by Azizi et al., the effect of antimicrobial compounds produced by L. brevis isolates against three bacteria, S. aureus (ATCC 25923), Listeria innocua (ATCC 33090), and Escherichia coli (ATCC 25922), was evaluated. The most susce-ptible and resistant bacteria to antimicrobial comp-ounds produced by Lactobacillus brevis were S. aureus and Listeria innocua, respectively (45). Jabberi et al. in 2017 evaluated the antimicrobial activity of L. brevis isolated from cottage cheese made in pottery against S. aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), S. typhimurium (ATCC 19430), and E. coli (ATCC 25922) using well diffusion method. The results of their study showed that the largest and smallest growth inhibition zones were observed against S. aureus and E. coli, respectively (46). In 2020, Hojjati et al. examined the antagonistic activities and safety properties of L. brevis isolated from traditional Iranian cheese. In this study, the antibacterial effect of L. brevis against pathogenic strains of S. aureus (ATCC 25923), Pseudomonas aeruginosa (PTCC 1707), S. typhimurium (PTCC 1609), and E. coli (ATCC 25922) was evaluated. The results showed that the most susceptible species were S. aureus, and the most resistant species was S. typhimurium (47).
The third part of this study was about the susceptibility of L. brevis to common antibiotics. The mechanism of how antibiotics affect bacteria is different. The antibiotics Vancomycin and Ampicillin destroy the cell wall, Tetracycline and Chloram-phenicol inhibit protein synthesis, and Penicillin affects cell permeability. In a 2011 report by Kirtzali-dou et al., the strains of L. brevis, L. plantarum, and Lactobacillus curvatus were resistant to Vancomycin (48). In a 2014 study by Shazali et al., L. brevis was resistant to Ciprofloxacin, Tetracycline, and Vanco-mycin (49). A study by Falah et al. in 2019 showed that L. brevis is resistant to Vancomycin and Gentamicin and is susceptible to Ampicillin, Chloramphenicol, and Kanamycin (50). Otaghsara et al. in 2020 reported that the L. brevis strain is resistant to Vancomycin and intermediate susceptible to the Tetracycline and Streptomycin antibiotics (51). In this study, the resistance of L. brevis isolated from cottage cheese was distinct to different antibiotics. The results show-ed that L. brevis isolates were resistant to Vancomycin and Gentamicin, intermediate susceptible to Kanam-ycin and Nalidixic acid, and susceptible to Erythro-mycin, Ampicillin, Tetracycline, and Chloramphenicol.
Microorganisms' isolation from local sources is an effective way to obtain valuable strains with unique characteristics. In this study, Lactobacillus brevis isolated from the Bazoft cottage cheese had good antimicrobial properties against pathogenic bacteria. Also, antibiotic evaluation in L. brevis is necessary from a consumer safety perspective and can be considered to replace the lost microbial flora of the gastrointestinal tract during antibiotic therapy.
We would like to express our gratitude and thanks to all those who helped us during this research.
This article was done without organizational financial support.
Conflicts of Interest
There is no conflict between the authors of the article.
Received: 2021/08/3 | Accepted: 2021/12/15 | ePublished: 2022/01/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |