BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1402-en.html


 , Maryam Koupaei2
, Maryam Koupaei2 

 , Horieh Saderi2
, Horieh Saderi2 

 , Seyed Mahmoud Amin Marashi3
, Seyed Mahmoud Amin Marashi3 

 , Zahra DehghanZadeh1
, Zahra DehghanZadeh1 

 , Parviz Owlia4
, Parviz Owlia4 


2- Department of Microbiology and Immunology, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran
3- Department of Microbiology, Qazvin University of Medical Sciences, Qazvin, Iran
4- Molecular Microbiology Research Center (MMRC), Shahed University, Tehran, Iran ,
Pseudomonas aeruginosa is a ubiquitous pathogen that causes opportunistic infections in humans (1). P. aeruginosa causes different disorders for example infection in the respiratory tract, urinary tract, skin and soft tissue, ʻ swimmers' ear ʼ and bacterial keratitis (2). Exotoxin A (PE) is a mono-ADP-ribosyltransferase family member with enzymatic properties (3). More than 90% of P. aeruginosa isolated from patients produce exotoxins A. High levels of exotoxin transcription are involved in severe infections (4).
Lactobacillus and Bifidobacterium are common components of ‘probiotic’ health foods, although their ability to elevate human health is controversial (5). The application of S. cerevisiae in the industry is wide since its ease of cultivation, achievement at the industrial level, and considered safe (GRAS) status (6). Saccharomyces cerevisiae var. boulardii has various remedial effects in gastrointestinal disorders, especially different types of diarrheas (7) such as food and traveler diarrhea, inflammatory bowel disease and acute gastroenteritis has been identified (8).
Research has shown that probiotics have been effective in the local treatment of P. aeruginosa wound infections (9). Al-Azzawi used lactobacilli (L. acidophilus and L. rhamnosus) in the role of a potential biotherapeutic factor (probiotic) for the treatment of P. aeruginosa infection (10). S. boulardii has beneficial effects through a mechanism independent of immune system modulation on the host’s gastrointestinal tract (11).
Finding alternative therapy for P. aeruginosa infections is a vital issue worldwide. The beneficial therapeutic effects of probiotics in inhibiting P. aeruginosa are well established today. The present study aimed to demonstrate the effect of probiotic yeast S. cerevisiae S3 supernatant and lysate on the expression of P. aeruginosa exotoxin A gene by using the real-time PCR method.
2.1 Strains and Growth Conditions
This research operated at the Molecular Microbiology Research Center (MMRC) of Shahed University. The native probiotic yeast S. cerevisiae S3 strain was isolated from fruits and dairy products with previously identified characteristics was used. S. cerevisiae was kept at -70°C in Potato Dextrose Broth (PDB) (Merck, Germany) with 20% glycerol. The bacterial strain used in this research was P. aeruginosa PAO1. P. aeruginosa has grown aerobically at 37°C in Brain Heart Infusion (BHI) agar (Merck, Germany) and kept at -70°C in BHI Broth, including 15% glycerol.
2.2 S. Cerevisiae Culture Condition
Several colonies of S. cerevisiae were inoculated to 20 mL of PDB medium (30°C, 16 h, 230 rpm). The 24-hour culture was applied to add 1000 mL of PDB. The medium was separated into different containers. The containers were incubated (30°C, 24 h, 120 rpm) and centrifuged (3000 g, 10 minutes) to assemble the supernatant and cell pellet.
2.3 Supernatant and Lysate of S. cerevisiae
The supernatant of S3 was acquired according to the Krasowska et al. method with a modification (12). A 0.22 μm filter was used for the filtration of supernatant. After that, 250 ml of overnight culture of S. cerevisiae was centrifuged (3000 g, 10 min). The supernatant was placed in the decanter for three hours with one- fifth volume of ethyl acetate. Every half hour ethyl acetate was changed. Finally, ethyl acetate was eliminated from the supernatant through a rotary evaporator (Hei-VAP Advantage ML, Heidolph, Germany). Approximately 45 mg of dry matter was prepared from one liter of yeast medium. A suitable amount of methanol was enhanced to the dry matter to prepare stock supernatant to reach a concentration of 327.68 mg/mL. The sediment obtained by centrifuging the culture medium in the former step was washed with distilled water and then dissolved again. In the next step, the yeast cells were lysed on the ice using a sonicator (Q125 Sonicator, Qsonica, USA). Every sonication step was done for 20 min (50-sec pulse on then 10-sec pulse off whit 100% amplitude). The lysed cells were centrifuged (15000 rpm, 4°C, 30 min). A 0.45 μm filter was used for filtration of lysate. A rotary evaporator was also used to remove water from the lysate. Finally, by increasing a suitable value of double-distilled water to the dried lysate powder, lysate stock with a concentration of 163.84 mg/ml was prepared.
2.4 Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
With the broth microdilution method in BHI Broth, the MIC was determined. Lysate and supernatant extracts were added separately to the microplate wells and BHI Broth (Merck, Germany) was added. The range of concentrations for both supernatant and lysate was 8192 to 16 μg/mL, and 105 CFU/mL bacteria were in each well. Well, without lysate and supernatant was used as positive, and without bacteria was applied as a negative control. Incubation conditions were included at 37°C for 20 hours. A well with the lowest concentration and did not show turbidity was introduced as MIC. For the measurement of MBC, the colony count was performed for wells without turbidity. Each well with the lowest concentration and showed a 99.9% decrease in the number of colonies was considered MBC. Because methanol was a supernatant solvent, MIC was performed to determine whether it had no inhibitory effect on bacterial growth.
2.5 Bacterial Culture Preparation to Determine Toxin Expression
First, 105 CFU/mL of bacteria from an overnighted culture of P. aeruginosa in BHI Broth was inoculated onto BHI containing 1024 μg/mL of supernatant and 8192 μg/mL lysate. The suspension was incubated until it reached the end of the logarithmic phase (37°C, 180 rpm). The pellet was removed after centrifugation (5000 g, 10 min).
2.5.1 Determination of Total mRNA
At first, the pellet was lysed with TE buffer. By applying the RNeasy Mini kit (Qiagen, Germany), total RNA was removed. The purity of RNA was determined by the NanoDrop device (NanoDrop One, Thermo Fisher, USA) .
2.5.2 Total cDNA Determination
QuantiTect Reverse Transcription Kit (Qiagen, Germany) was applied to remove genomic DNA and synthesize the cDNA. Two microliters of gDNA without buffer were added to 12 µl of RNA and placed at 42°C for 2 minutes. Then immediately placed on ice. RT Buffer, Random Primer and RT enzyme were added to the above mixture and placed at 42°C for 30 minutes. finally placed at 95°C for 3 minutes to inactivate the enzyme. Two microliters of cDNA were mixed with 18 µl of water, which was used as a template.
2.5.3 Real-time PCR
The real-time PCR was done by Quantitect SYBR Green PCR Kit (Qiagen, Germany) and Rotor-Gene Q (Qiagen, Germany). The primer sequence showed in Table 1. Sequences were designed using AlleleID 6 software. Real-time PCR was performed according to the following protocol: one cycle of 95°C for 15 minutes, after that 40 cycles of 94°C for 15 seconds, 57°C for 30 seconds, 72°C for 30 seconds and a terminal extension at 60°C for 15 seconds.
Table 1. Primers used for quantitative real-time PCR
| Reverse primer | Forward primer | gene |
| 5′CCTCGTCGCCTTCCTTCAC3′ | 5ˊGTTGATGAAGATGACCAGGCA3́ | recA |
| 5́ GTAGCCGACGAACACATAGC3́ | 5́ GTCGGGTTCCTGGTCCTG3′ | exoA |
2.5.4 Determination of toxin expression
After the amplification, the melting curve was compared with the curve obtained from genomic DNA. In fact, this was done to determine that all primers have the same rate of elongation and performance. For normalization, the expression of the housekeeping recA gene was applied. The 2-ΔΔCt method was used for determining Relative gene expression. Distilled water was used for negative control.
3.1 The results of MIC and MBC
The MIC value of the supernatant for P. aeruginosa was obtained to be 2048 μg/mL. The MBC was 2048 μg/mL. The lysate had no bacteriostatic and bactericidal effects on P. aeruginosa. Methanol also did not affect inhibiting P. aeruginosa growth.
3.2 The results of Real-time PCR
The exoA Gene expression in P. aeruginosa was measured using real-time PCR method. Melting curves were drawn to confirm the lack of contamination and primer dimer. If one peak was present in the melting curve, the reaction’s correctness was considered.
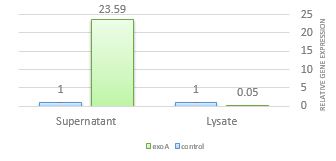
Table 2 displays the outcomes of the exoA cycle threshold (Ct) analysis in the presence of lysate and supernatant. The results showed that the yeast supernatant could not decrease the expression of the exoA in P. aeruginosa. The lysate extract decreased the expression of the exoA (fig. 1).
Table 2. Cycle threshold (Ct) results for exoA.
| ratio | Mean ΔΔCt | CT Mean±SD | Gene | ||
| 1/00 | 0/00 | 22.69±1.53 | recA | Lysate | |
| 0/05 | 4/21 | 26.16±0.55 | exoA | ||
| 1/00 | 0/00 | 17.37±0.05 | recA | Supernatant | |
| 23/59 | -4/56 | 17.24±0.71 | exoA | ||
ΔΔCt was calculated as: ΔCt (test) - ΔCt (calibrator). Ratio = efficiency – ΔΔCt.
Figure 1. Effect of supernatant and lysate extracts of S. cerevisiae S3 on the expression of exoA. Compared with control, exoA gene expression decreased only in the presence of lysate.
P. aeruginosa is a prevalent cause of various infections such as nosocomial pneumonia, UTI, and surgical infections (13). P. aeruginosa contains various virulence factors that have significant role in its pathogenicity such as Exotoxin A (14). S. cerevisiae var. boulardii have activities that can help improve the different functions of its host (15). In the present study, the effect of supernatant and lysate of S. cerevisiae S3 on the exoA gene expression was indicated. In our study by using real-time PCR was shown that probiotic lysate had a reducing effect on the expression. Supernatant had not decreased effect on the expression of the exoA gene.
Lactobacilli are the most well-known probiotics that are widely used in different societies. They can act against pathogens in various ways, such as producing inhibitory compounds (organic acids, protein compounds and hydrogen peroxide) and improving immune system (16). Ramos and colleagues found the characteristics of Lactobacillus plantarum culture supernatants (LAPS) and showed therapeutic effect on the treatment of chronic wound infection (17). Ogawa et al. showed the Lactobacillus bactericidal effect on STEC. They believed the lactic acid generation and pH reductive effect on the survival of pathogens (18). Two bacteriocins of L. plantarum prevented the growth of P. aeruginosa (19). One study examined the effect of S. cerevisiae on E. coli O157:H7 and suggested that ethanol secreted by probiotics may have an antagonistic role in the small intestine (20). Kiymaci et al. demonstrated the effect of lactic acid secreted by Pediococcus acidilactici on the virulence factors of P. aeruginosa (21). Fakruddin et al. used S. cerevisiae IFST062013 whole cell, supernatant of culture, and lysate of the cell to determine the growth rate of P. aeruginosa. Cell lysate had the best inhibitory effect (22). Similarly, we used the supernatant and lysate of yeast probiotics. In our study lysate just could inhibit the exoA gene expression. It is unclear why supernatant had no inhibitory effect on gene expression and more studies are required to understand it.
In contrast, the positive effects of probiotics on pathogens sometimes, we do not see an acceptable effect of probiotics in inhibiting pathogens, for example in a study conducted by Cho et al. P. aeruginosa co-cultured with Lactococcus lactis in the presence of porcine gastric mucin. They were not found an improving effect on the P. aeruginosa strains (23). In our study, we found supernatant of S. cerevisiae caused increasing in the expression of the exoA gene and only its lysate decreased gene expression. Therefore, we recommend further studies on the content of the supernatant and its effect on the pathogen.
The anti-diarrheal effects of some probiotics are anticipated from their antitoxin properties (7). Asahara et al. conducted experiments showing that Bifidobacterium could prevent EHEC verotoxin production (24). Carey and colleagues applied different types of probiotics against the expression of the toxin gene in EHEC and demonstrated that the level of expression reduced by the production of SCFAs (25). The cytotoxic effects of Bifidobacterium and lactobacilli on C. difficile have been demonstrated (26, 27). Bacillus clausii also produced a protease that prevented the cytotoxic effects of C. difficile and B. cereus (28). Exotoxin A is one of the toxins secreted by P. aeruginosa. Inhibition of exotoxin A production can play an important role in reducing the pathogenicity and destructive effects of P. aeruginosa. In this study, the lysate reduced the exoA gene expression. It is recommended that more studies be performed to determine the effect of this probiotic on other secretory toxins of this pathogen.
In a similar study conducted by DehghanZadeh et al., The effect of supernatant and yeast lysate of S. cerevisiae on the expression of two virulence factors, elastase and alkaline protease of P. aeruginosa, was investigated. Their study shows the excellent inhibitory effect of lysate on these two genes (29). In the present study, lysate also had a significant inhibitory effect on inhibiting the expression of exotoxin A gene.
S. boulardii has the ability to produce products such as organic acids, vitamins, essential enzymes and metabolites such as vanillic acid, phenyl ethyl alcohol and erythromycin (30). To better understand how S. cerevisiae is effective in inhibiting P. aeruginosa and reducing the expression of virulence factor genes, it is recommended to determine the components and compounds of each of the supernatants and lysates. Finally, the results of this study confirm the inhibitory effects of S. cerevisiae probiotic yeast on the expression of the exotoxin A gene in P. aeruginosa. The more accurate studies on probiotic properties, the better can use them for treatment options. So, the authors suggest designing other tests for comparing properties of probiotics on P. aeruginosa.
According to the present study, the lysate of S. cerevisiae probiotic yeast could reduce the exoA gene expression and supernatant increase it. Since exotoxin A is an important virulence factor in P. aeruginosa, probiotics can prevent it.
The authors thank Hadis Fathizadeh for her suggestions and guidance.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ZG contributed to: conceptualization, project administration, data curation, methodology. MK contributed to: project administration, writing: original draft preparation, review & editing. HS contributed to: writing review & editing, and validation. SMAM contributed to: methodology, supervision. ZD contributed to: conceptualization, project administration. PO contributed to: conceptualization, supervision. All authors read and approval the final version of the manuscript.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2022/10/25 | Accepted: 2023/03/28 | ePublished: 2023/06/26
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |