BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1307-en.html
2- Department of Microbiology, School of Basic Sciences, Kazerun Branch, Islamic Azad University, Kazerun, Iran ,
Introduction
Cancer is a complex disease that affects cells and disrupts the rate of cell proliferation and differen-tiation (1, 2). Usually, a secondary tumor is formed at the time of diagnosis, which is a late diagnosis and is the cause of high cancer mortality (1). In the Middle East, breast cancer accounts for 32% of cases of women’s cancers and is the first cause of women’s death aging 40 to 45 years old (1, 3). At the same time, prostate cancer is the most common malignant tumor in men. Generally, one in six men will develop prostate cancer in their lifetime (4). Breast cancer and prostate cancer are the most common causes of death in Iranian women and men, respectively (5). Also, bla-dder cancer is a common disease. In men, its incidence is 2 to 2.5 times more than women; how-ever, it is also common in women who smoke (6). Conventional therapies, such as chemotherapy and radiation therapy, have low survival rates due to tumor prog-ression, resistance to treatment, and non-specific tre-atment of tumors (7). Scientists are trying to use technology to introduce new treatments for cancer. Various applications of bacteria have been investiga-ted so far (7). Natural compounds derived from them (including bacterial pigments) are reported to have anti-cancer and antioxidant activity, and have unique cancer treatment mechanisms (7).
The most important feature of Pseudomonas aeruginosa is the production of the blue-green pigment of pyocyanine (8).
This pigment has an immunostimulatory effect on cancer patients and is related to cell apoptosis having great potential to inhibit cell proliferation. Hence, due to the action of superoxide dismutase and catalase, it generates a large amount of reactive oxygen species (ROS) (7). These results support the cytotoxicity of pyocyanine in cancer cells. These mechanisms have led to better cancer treatment strategies (9). Also, the pyocyanine has stimulatory activities on IL-2 cytokine such that low concentrations of pyocyanine exacer-bated the responses of T lymphocytes (production of IL-2) and B lymphocytes (differentiation to immune-globulin-producing cells), whereas high concen-trations of this pigment had inhibitory effects (10). IL-2 is a pro-inflammatory cytokine that is produced in T cells and participates in the stimulation of immune responses (11). Eventually, since the pyocyanine has immunostimulatory effects and considering that this pigment induces the transcription of inflammatory cytokines and stimulates the secretion of IL-2 from blood cells; the use of the immune system in cancer treatment is a promising approach and it is hoped that with the advancement of studies in this field, a new dimension will be added to cancer treatment (12, 13). This study aims to evaluate the ability of different clinical isolates of P. aeruginosa to produce pyocya-nine and the in vitro immunostimulatory effe-cts of pyocyanine on the IL-2 secretion from PBMCs in patients with breast, prostate, bladder cancers, and healthy control group.
Materials and Methods
Isolation and Identification of Pseudomonas aeruginosa from Clinical Samples
In this cross-sectional descriptive study, after approval in the committee for ethics in biomedical research of Kazerun Branch, Islamic Azad University, being presented with the code IR.IAU.KAU.REC-.1399.-031, a total of 30 P. aeruginosa isolates were collected from different clinical specimens such as urine, blood, burn, sputum, and wound infections of patients admitted to Namazi Hospital in Shiraz from October to December 2019. All isolates were identi-fied as P. aeruginosa using gram stain and biochemical charac-terization (14). The isolates were randomly eva-luated to determine the rate of pyocyanine produc-tion. To maintain the bacteria, the strain was inocula-ted in the broth medium of tryptic soy broth (Quelab, Canada) containing glycerol, and then stored at -70°C (15).
Extraction of Pyocyanine from Pseudomonas aeruginosa Isolates
All bacterial isolates were cultured on Pseudomonas agar culture media (Quelab, Canada), and incubated at 37°C for 48 hours. The positive isolates in the production of pyocyanine showed blue-green pig-ments. Then, chloroform (Merck, Germany) was pour-ed on the culture medium (1:2 ratio), then vortex (Labinco, Netherlands) vigorously for 1 minute. The culture was centrifuged at 5000 rpm (Hettich, Germany) for 10 minutes, and formed two organic and aqueous phases. The supernatant was separated and pyocyanine was extracted. Then, added 1 mL of 0.1M HCl (Merck, Germany) to observe pink to deep red color. The pigment was purified by repeated addition of the chloroform solvent (16).
Extracted pyocyanine was poured into a watch glass (Schott Duran, Germany) and kept for 24 to 48 h under a hood (BioBase, Iran) to completely remove chloroform. Finally, the dried pigment was separated by a sterile lancet (Azmaplast, Iran) and stored in the refrigerator in powder form for later stages. The concentration (μg/mL) of the pigment was deter-mined by measuring the optical density at a wavele-ngth of 520 nm using a spectrophotometer (Unico, USA) (16, 17).
Patients and Study Population
Due to the rapidly increasing prevalence of breast, prostate, and bladder cancers, and the urgent need for their treatment, the sampleas were selected from among the patients diagnosed with these cance-rs.Twenty-one patients with breast (n=7), prostate (n=7), and bladder (n=7) cancers admitted to the Cancer Center of Shiraz Namazi Hospital were included in this study from August to December 2019. The reason for choosing 7 samples per group was that the number was statistically acceptable. Seven hea-lthy volunteers were recruited as a control group. There was no history of cancer in this group. The age range (46-65 years old) and male to female ratio were similar in studied cancer patients and healthy controls. Patient and control groups were matched for age and gender. Five mL fresh blood was obtained from cancer patients and healthy individuals by venous puncture and collected in sterile tubes containing EDTA. The sample was sent to the laboratory for the isolation of peripheral blood mononuclear cells (PBMCs). The specifications of breast, prostate, and bladder cancer patients were shown in table 1.
Table 1. Specifications of breast, prostate, and bladder cancer patients
| Metastasis | Stage | Family history | Age | Sexuality | No. | |
| Breast cancer patients (n=7) | ||||||
| Yes | III | No | 59 | Woman | 1 | |
| Unknown | II | No | 53 | Woman | 2 | |
| No | I | Yes | 46 | Woman | 3 | |
| Unknown | II | No | 55 | Woman | 4 | |
| Unknown | II | Yes | 63 | Woman | 5 | |
| Unknown | II | No | 57 | Woman | 6 | |
| Unknown | II | No | 50 | Woman | 7 | |
| Prostate cancer patients (n=7) | ||||||
| Unknown | III | Yes | 72 | Man | 1 | |
| No | II | Yes | 59 | Man | 2 | |
| No | II | Yes | 56 | Man | 3 | |
| No | II | No | 62 | Man | 4 | |
| Unknown | III | No | 70 | Man | 5 | |
| No | I | Yes | 57 | Man | 6 | |
| No | I | No | 65 | Man | 7 | |
| Bladder cancer patients (n=7) | ||||||
| No | I | No | 49 | Man | 1 | |
| No | I | No | 58 | Man | 2 | |
| No | II | No | 60 | Woman | 3 | |
| No | I | Yes | 39 | Man | 4 | |
| No | I | Yes | 44 | Man | 5 | |
| No | I | No | 50 | Man | 6 | |
| No | I | Yes | 45 | Woman | 7 | |
Separation of PBMCs from Patients with Cancer and Healthy Controls
Separation of PBMCs from patients with cancer and healthy control samples was performed using the ficoll density gradient method (18). The blood sample was transferred to a 50 mL tube and diluted 1:1 ratio with PBS (Merck, Germany). Then, diluted blood samples were slowly added to the Ficoll (Inno-Train, Germany). The samples were centrifuged at 400 rpm for 20 minutes at 20°C (Hettich, Germany). The PBMCs (cloudy layer) were collected from the diluted plasma/ficoll interface using a serological pipette and the cells were placed into a sterile 50 mL tube. Again, the cells were washed by adding PBS and centrifuged at 330 rpm for 10 minutes (19).
Cultures of PBMCs Isolated from Patients with Cancer and Healthy Controls
After the separation was performed, 100 µL PBMCs isolated from the patients with cancer and the healthy control group was transferred to a 25 cm2 flask (Jetbiofil, Canada) containing RPMI 1640 medium (Biosera, France) and 20% fetal bovine serum (FBS) (Biosera, France). The flask was placed in a 5% CO2 incubator (Lab Tech, South Korea) at 37°C and 98% humidity. After 48 hours, the culture medium was replaced and the cells were passaged (20).
In order to harvest the cells attached to the bottom of the flask, the medium was washed thoroughly and slowly with PBS. Then, added the trypsin enzyme (Merck, Germany) to the flask and placed in the incubator at 37°C for 2 to 5 minutes. After pipetting, the cells isolated from the flask floor were transferred to a 15 mL sterile falcon tube. The tube containing the cells was centrifuged for about 5 minutes at 1200 rpm. The supernatant was evacuated to the cells and the new medium was added. The cell suspension was split into several new flasks. 20 μL cell suspension was mix-ed with 20 μL trypan blue (Sigma, USA). Then, live cells (cells that do not penetrate the color) were counted in the 25th hemocytometer (HBG, Germany) (20).
Effect of Pyocyanine on the Secretion of IL-2 from PBMCs of Patient and Control Groups
Half of the PBMCs of cancer patients and healthy controls were treated with pyocyanine (0.5 μg/mL), and the rest of them remained without treatment. Then, the supernatants were removed and the concentration of IL-2 was determined by ELISA using a commercial human IL-2 ELISA kit (Thermo Fisher Scientific, Australia), according to the manufacturer’s instructions.
Statistical Analysis
The secretion of IL-2 from the treated and untreated cells with pyocyanine was compared by paired sample's T-test. Also, statistical analysis of the samples was done using SPSS version 22 (SPSS Inc., Chicago, IL., USA). All results were expressed as mean ± standard error (SE). A p-value of 0.05 or less was considered statistically significant.
Results
Selection of the Highest Pyocyanine Producer Isolate
Among clinical isolates, isolate 11 (from a wound source) was the highest pyocyanine-producing agent, and this isolate was used for testing in the same sample. Table 2 shows the concentrations of pyocy-anine produced by Pseudomonas aeruginosa isolates.
Table 2. The concentrations of pyocyanine produced by P. aeruginosa isolates
| Concentration of pyocyanine (µg/mL) | OD | Source | No. Isolates |
| 3.41 | 0.20 | Urine | 1 |
| 3.24 | 0.19 | Urine | 2 |
| 1.36 | 0.08 | Wound | 3 |
| 7.17 | 0.42 | Blood | 4 |
| 1.70 | 0.10 | Urine | 5 |
| 2.90 | 0.17 | Burn | 6 |
| 3.58 | 0.21 | Burn | 7 |
| 4.60 | 0.27 | Blood | 8 |
| 5.80 | 0.34 | Blood | 9 |
| 3.24 | 0.19 | Blood | 10 |
| 9.90 | 0.58 | Wound | 11 |
| 1.02 | 0.06 | Urine | 12 |
| 3.41 | 0.20 | Urine | 13 |
| 7.85 | 0.46 | Blood | 14 |
| 9.38 | 0.55 | Burn | 15 |
| 5.12 | 0.30 | Urine | 16 |
| 1.19 | 0.07 | Sputum | 17 |
| 1.53 | 0.09 | Wound | 18 |
| 5.63 | 0.33 | Blood | 19 |
| 6.82 | 0.40 | Urine | 20 |
| 1.19 | 0.07 | Urine | 21 |
| 7.68 | 0.45 | Blood | 22 |
| 2.90 | 0.17 | Blood | 23 |
| 1.36 | 0.08 | Urine | 24 |
| 1.53 | 0.09 | Sputum | 25 |
| 7.85 | 0.46 | Burn | 26 |
| 6.65 | 0.39 | Urine | 27 |
| 5.63 | 0.33 | Sputum | 28 |
| 2.21 | 0.13 | Blood | 29 |
| 7.68 | 0.45 | Urine | 30 |
Comparison of IL-2 Secretion from PBMCs of Patients and Control Groups Before and After Treatment with Pyocyanine
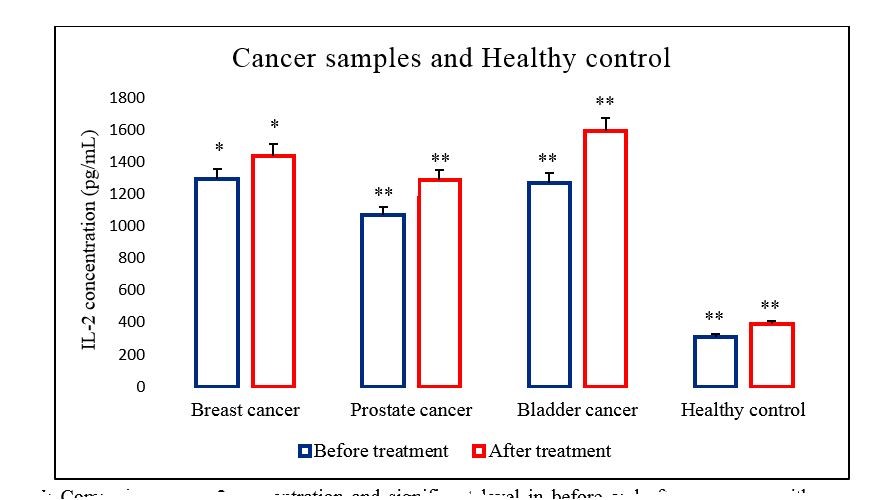
The ELISA method was used to compare the secretion levels of IL-2 in PBMCs of cancer patients (n=21) and healthy control samples (n=7) before and after treatment with pyocyanine pigment (Figures 1-4). In all groups, after treatment with pyocyanine, the secretion level of IL-2 of PBMC was higher than that of pretreatment with this pigment.

Figure 1. IL-2 production from PBMCs of breast cancer patients (n=7) before and after treatment with pyocyanine
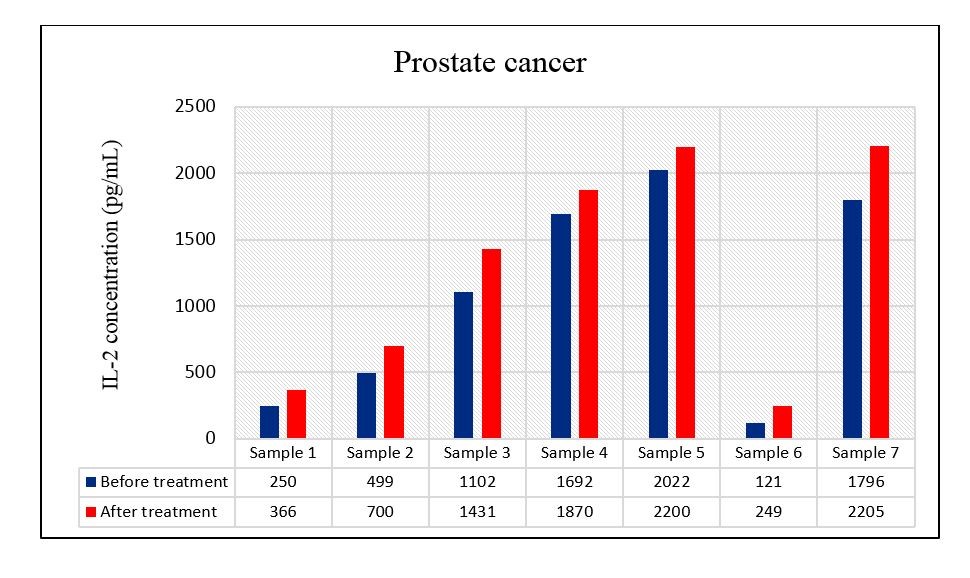
Figure 2. IL-2 production from PBMCs of prostate cancer patients (n=7) before and after treatment with pyocyanine
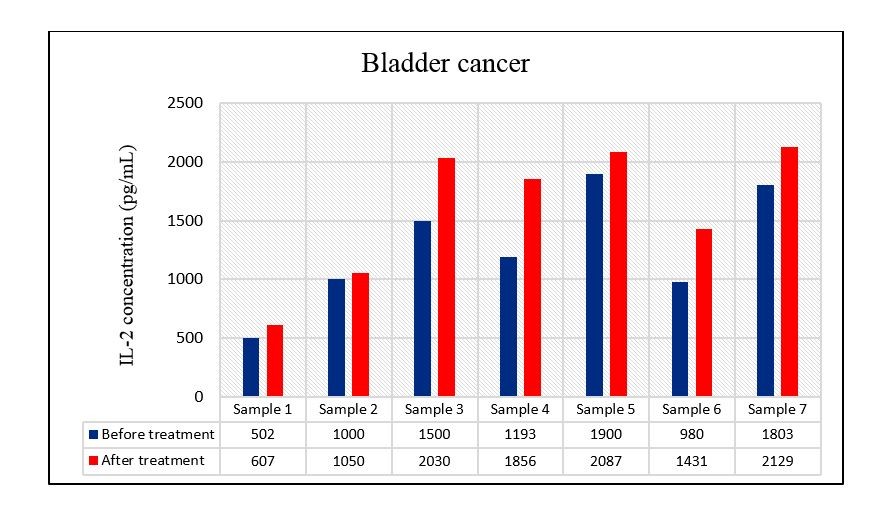
Figure 3. IL-2 production from PBMCs of bladder cancer patients (n=7) before and after treatment with pyocyanine
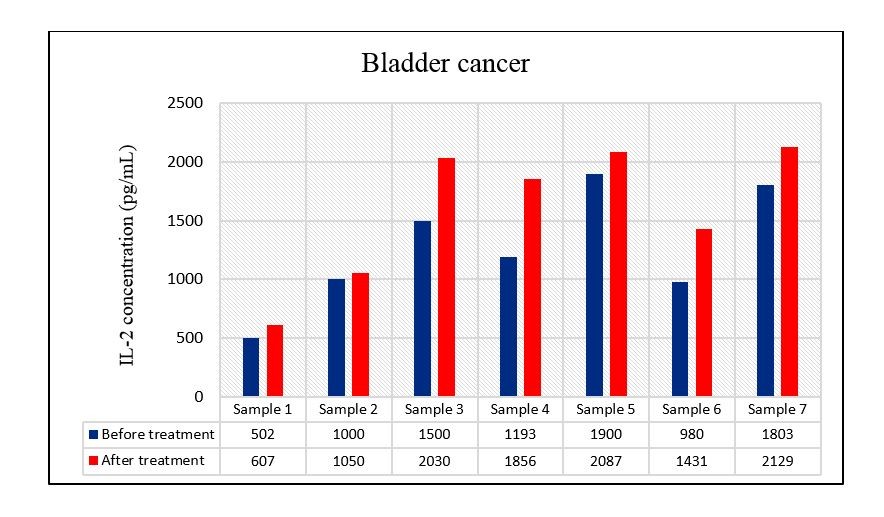
Figure 4. IL-2 production from PBMCs of healthy individuals (n=7) before and after treatment with pyocyanine
The secretion of IL-2 from PBMCs of breast cancer patients was significantly increased after treatment with pyocyanine in comparison with before treatment with this pigment with a mean of 1440 ± 252.2 pg/mL vs 1296 ± 246.56 pg/mL (P=0.032) (Figure 5).
The secretion of IL-2 from PBMCs of prostate cancer patients was significantly higher in the treated cells with pyocyanine than that in untreated cells with this pigment with a mean of 1288.71 ± 320.93 pg/mL vs 1068.86 ± 298.27 pg/mL (P=0.002) (Figure 5).
The IL-2 secretion level from PBMCs of bladder cancer patients was significantly increased after treatment with pyocyanine in comparison with before treatment with this pigment with a mean of 1598.57 ± 223.26 pg/mL vs 1268.29 ± 188.54 pg/mL (P=0.009) (Figure 5).
The secretion of IL-2 from PBMCs of healthy control individuals was significantly higher in the treated cells with pyocyanine than that in untreated cells with this pigment with a mean of 392.71 ± 52.17 pg/mL vs 311.29 ± 56.74 pg/mL (P=0.004) (Figure 5).
Therefore, in breast cancer, prostate cancer, bladder cancer, and healthy controls, the increase in IL-2 concentration after treatment with pyocyanine was 11.1%, 20.5%, 26%, and 26.1%, respectively (Figure 5).
Figure 5. Comparison of IL-2 secretion from PBMCs of cancer samples and healthy control before and after treatment with pyocyanine. P<0.05 (*) and P< 0.01 (**).
Discussion
The bacterial pigments have immunostimulatory effects on the immune system by the release of cytokines in cancer patients (21). Despite the importance of the immune system in response to cancer treatment, there has been no report on how to stimulate the immune system with the pyocyanine pigment of Pseudomonas aeruginosa.
Based on the results of this study, a low concen-tration of pyocyanine stimulates the IL-2 secretion by PBMCs in cancer patients in vitro. Imm-unotherapy using IL-2 is the most effective anti-cancer treatment (12). The mechanism of the action of pyocyanine on IL-2 production is still unclear. Pigment probably stimulates the patient’s immune system (22). In 2011, research conducted by Rada and colleagues found that pyocyanine can induce the tran-scription of inflammatory cytokines and epider-mal growth factor receptors, as well as IL-1, IL-6, IL-8 cytokines, and TNFα (alpha tumor necrosis factor) (22). Perhaps certain bacterial pigments (such as pyoc-yanine) activate signal transduction pathways through Toll-like receptors (TLR) in PBMC, thereby stimulating the secretion of cytokines including IL-2 (23). The immunostimulatory effect of pyocyanine depends on the concentration of pyocyanine tested (24).
In one study, the inhibitory and stimulating activity of pyocyanine on IL-2 secretion was tested (10). The results were different and the cellular response was related to the concentration of pyocyanine (10). Low concentrations (0.1, 0.2, 0.5 μg/mL) of pyocyanine will increase the response of T lymphocytes (IL-2 production) and B lymphocytes (differentiation of immunoglobulin-producing cells), while high concen-trations of this pigment (1 μg/mL) has an inhibitory effect (10). Similarly, in this study, it was observed that after treatment with a low concentration of pyoc-yanine (0.5 μg/mL) in vitro, the IL-2 secreted by the PBMC of breast cancer, prostate cancer and bladder cancer patients increased.
IL-2 is an activator of cytotoxic T cells (CTL) and natural killer cells (NK cells) (10). NK cells destroy the target cell by secreting their granules (enzymes and proteins) and designing apoptosis (25). NK cells are transformed into lymphokine-activated killer cells (LAK cells) under the action of IL-2 (25). LAK cells have higher apoptotic activity and can destroy different tumor cells (25).
In studies on the role of IL-2 in cancer treatment, similar studies have been conducted on the positive effects of IL-2 in the recovery and treatment of patients with kidney, breast, prostate, and bladder cancer (26-30). Metastatic melanoma and renal cell carcinoma treated with high-dose Interleukin-2 (30). In advanced melanoma, high-dose IL-2 injection is effective in 15% to 20% of patients (30). The patients with metastatic breast cancer were treated with IL-2 and this cytokine increased the number of NK cells and cell lysis function in the body (29). Freytag and colleagues demonstrated the IL-2 gene therapy strategy for local and metastatic prostate cancer in 2007 (28). The result was a 50% reduction in tumors in patients after IL-2 treatment (28). Askeland and colleagues in 2012, showed the function of IL-2 in increasing the NK cells in patients with bladder cancer, and the systemic result of IL-2 in reducing the size of the tumor, tumor growth, and survival time of cancer patients (27). These results indicate that IL-2 has an important role in reducing inflammation in the tumor environment and can have a very positive effect during regular periods, which can indicate a reduction in tumor volume growth. It is recommended in future research, increase the size of the statistical community as much as possible.
Conclusion
The results of this study indicate that treatment with pyocyanine pigment can increase the IL-2 concen-tration of PBMCs in patients with breast, prostate, bladder cancer, and healthy controls. Low concen-trations of pyocyanine may activate the host's imm-une response. This pigment stimulates the secretion of IL-2 by activating the TLR signal transduction pathway in PBMC and inducing the transcription of cytokines. With the unique properties of pyocyanine and the human desire for biology and low-risk treatments, we hope that it can broaden our understanding of immunobiology and recognize the immunostimulatory effects of various bacterial pigments on cancer patients. Although this study is the first step to identify the immunostimulatory effects of pigments in vitro, the anti-tumor effects of pyocyanine in experimental animals should be further studied. Also, more efforts are needed to determine the mode of action and the potential of using the pigment in the stimulation of PBMCs in cancer patients for the anti-cancer IL-2 secretion.
Acknowledgements
This article is the result of a part of the master's thesis of microbiology from the Islamic Azad University, Kazerun Branch. The project was approved by the committee for ethics in biomedical research of Islamic Azad University, Kazerun Branch (IR.IAU.KAU.-REC.1399.031). The authors thank all those who helped them writing this article.
Conflicts of Interest
The authors declared no conflict of interests.
Received: 2021/03/27 | Accepted: 2021/05/2 | ePublished: 2021/06/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |