BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1222-en.html
2- Department of Pathobiology, Faculty of Veterinary Medicine, Shahrekord University, Shahrekord, Iran ,
Introduction
Herbal medicines are a group of herbs that contain a number of important compounds for medicine and pharmacy (1). Effective herbal remedies have attr-acted global attention because of their easy access and low side effects (2). Endophytes have been found to reside in the host tissues without apparent damage or signs of disease promoting plant growth and health (Symbiotic effect). Also, they provide a high resistance to a variety of diseases. Endophytes are powerful producer of secondary metabolites (3). Antimicrobial metabolites extracted from endophytes belong to variety structural classifications, including peptides, alkaloids, phenols, steroids, flavonoids, terpenoids, etc. (4). Common endophytes including a variety of bacteria (actinomycetes) and fungi are generally isolated from plants (5). These microorganisms have antifungal and anti-bacterial properties and prevent pathogens from entering the plant (6). New antibiotics (antimycotics), and anticancer compounds are just few examples of endophytes isolates (7).
Due to the increasing of the antibiotic resistance of pathogenic microbes, some of antibiotics can be substituted by endophytes and they may provide the alternative sources of bioactive compounds and probiotics for treatment of some bacterial and fungal diseases (8).
Escherichia coli is the leading cause of death worldwide with a wide range of diseases. Each year, it causes widespread infant mortality due to diarrhea and septicemia (9). E. coli strains are generally resistant to a number of medications (10).
Various Staphylococcus aureus isolates have different responses in terms of antibiotic sensitivity or resistance. Scientific reports have suggested that methicillin-resistant strains of S. aureus (MRSA) indi-cating changes in the epidemiology of this bacterium.
The ability of some Allium species has been shown to reduce risk factors such as elevated serum cholesterol, elevated LDL, increased platelets, hyper-tension, and other factors by in vitro studies (14,15). Allium jesdianum belongs to the Alliaceae family which grows in the west and southwest of Iran and is traditionally effective in treating and alleviating rheumatic and gastrointestinal pain, renal stone excretion as well as colds and abdominal pain. It has analgesic and pain relieving properties (16,17).
In this study, the bacterial endophytes were firstly isolated from A. jesdianum and then their antifungal and anti-bacterial properties against some pathogenic bacteria and fungi were investigated.
Materials and Methods
Isolation of Endophytic Bacteria
Fresh samples of A. jesdianum (various parts of the herb including stem, leaf, onion and flower) were collected randomly from Chaharmahal and Bakhtiari province, Iran, in the spring 2019. In this clinical trial study, a minimum of 30 and a maximum of 50 fresh samples of A. jesdianum were used to isolate a maximum of 10 bacterial endophytes to evaluate their antibacterial and antifungal properties. Samples of A. jesdianum were transferred to the microbiology labor-atory at Shahrekord University, Chaharmahal and Bakhtiari, Iran. The genus and species of this plant were confirmed taxonomically by the Department of Botany.
The plant was cleaned, sterilized and dried. The stems, leaves, onions and flowers of A. jesdianum were separately placed in 70% ethanol for 2 minutes. They were then immersed in 5.3% sodium hypo-chlorite solution and 75% ethanol for 5 minutes and 30 seconds, respectively (18). Subsequently, the ingredients of the plant were washed with sterile distilled water and then dried. The final distilled water was then cultured on a nutrient agar as a control. For the isolation of endophytic bacteria, stems, leaves, flowers and onions were cut by a sterile scalpel and transferred into agar media containing yeast extract (Merck, 64271, Germany) or YEA (Yeast Extract Agar: Yeast Extract 5g/L, Glucose10g/L, Agar 16g/L) and the pepton agar medium (Pepton Agar: Pepton Water 15g (Difco, 1807-17-4)/L, Agar 16g/L (Quelab, 420223). All culture media and the control plates were directly incubated at 35°C for 4-7 day. After the incubation period, the endophytes were developed in the plates and labeled at random for better identification. Each colony was examined macroscopically on an individual basis (19). The morphology of the purified colony of the endophytic bacteria was recorded in terms of shape, color and size. Bacterial identification were done by catalase and oxidase tests, gram staining and biochemical assays (20,21).
Bacterial and Fungal Strains
Respectively, S. aureus (ATCC: 25923) and E. coli (ATCC: 25922) as standard strains were collected from Shahrekord University of Medical Sciences, Iran. Biochemical and morphological identification were carried out to confirm the prepared strains (21). C. albicans and T. mentagrophytes, as fungi, were obtained from Department of Mycology, University of Tehran, Iran and were re-cultured in the sabouraud dextrose agar medium (Merck, Germany).
Investigation of Antibacterial and Antifungal Properties of Agents Within the Endophytic Bacteria Through the Drip Method by Chloroform (DMC)
Three to four entirely similar colonies of endophyte bacteria were separately cultured in a Pepton water medium (Difco, 1807-17-4) and incubated at 37°C for 18 to 24 hours. A total of 50 µL of the prepared suspension of each endophyte was placed on Pepton Agar and Yeast Extract Agar medium (YEA, Merck-, 64271, Germany) and incubated (37°C for 18-24 hours). In the next step, the surface of the endophytic colonies were coated by chloroform for 20 to 30 minutes until chloroform completely evaporated (19).
Three to four completely identical colonies of each pathogens (S. aureus ATCC 25923, E. coli ATCC 25922 and C. albicans) were cultured separately in a Tryptic Soy Broth (TSB) medium and incubated for 24 hours at 37°C. After the incubation period, 200 µL was collected from the TSB medium (at a concentration of 0.5 McFarland) and transferred into tubes containing 10 mL of semisolid BHI culture medium (Himedia, 400-086) in 37 to 40°C.
The contents of each tube were added to the surface of the YEA and PA medium containing endophytic bacterial colonies, so that the surface of the plate was completely covered with BHI medium and incubated at 37°C for 18 to 24 hours.
A total of 1-2 pure cultured colonies of T. mentagrophytes were also removed by a needle and cultured 1 cm away from the endophyte bacterial colony at four corners of the plate. Following this period, the diameter of the growth inhibition zone of the pathogens formed around each endophytic colony was measured and recorded in millimeters. Each endophytic isolate was tested separately. Also, before performing the aforementioned steps, to find the antibacterial and antifungal properties of the endophytes, the antibiogram of pathogens was examined and confirmed in the standard tables. The experiments were repeated in triplicate and the standard deviation of the mean (SD) as well as standard error of the mean (SEM) was calculated and documented (19).
Investigation of Antibacterial and Antifungal Properties of Endophytic Bacteria by Secretory Metabolite Test (SMeT)
Three to four colonies of each endophytic bacterium were cultured separately in a LB broth (Luria Bertani Broth, Merck, 110285, Germany) and incubated at 37°C for 18-24 hours.
The standard pathogens including S. aureus, E.coli, and C. albicans were cultured in LB medium and incubated. A total of 200 µL of LB medium, at the appropriate concentration of 0.5 McFarland, was added to 15 mL of YEA medium, mixed and poured into sterile plates. Without wasting time and before the solidification of the culture medium, the sterile aluminum cylinders (0.5 cm in diameter and 1 cm in height) were inserted into the plates with appropriate spacing. The YEA culture medium was prepared and autoclaved and poured in the plates. Before solidification of the culture medium, the sterile aluminum cylinders were inserted into the plates. A total of 1-2 pure colonies of T. mentagrophytes were removed by a straight needle and cultured in 4 corners of the plate, containing YEA medium. A total of 1 mL of LB broth (Merck, 110285, Germany) containing cultured endophyte bacteria at the appropriate concentration (standard 0.5 McFarland) was trans-ferred to a sterile microtube. The microtube contents were centrifuged (Sigma, serial no. 103286) at 10,000 rpm for 15 minutes. After filtration of the centrifugal supernatant, 100 µL was removed and carefully poured into each cylinder. Sterile water was also considered as a control group. The plates were incubated at 37°C for 24 h. These steps were per-formed separately for each endophytic strain as well as pathogens. After incubation, the created diameter of the pathogens growthless zone (S. aureus ATCC: 25923, E.coli ATCC: 25922 and C. albicans) around each cylinder was measured and recorded in millimeters. Growth in T. mentagrophytes was also evaluated and compared to the controls. The experiments were repeated and the standard devia-tion of the mean (SD) as well as standard error of the mean (SEM) were calculated and documented (22).
Statistical Analysis
Data was statistically analyzed by SPSS 22 (SPSS Inc., Chicago, IL., USA). The Antimicrobial effect of A. jesdianum endophytes on the pathogens (S. aureus, E. coli & C. albicans) was calculated in both methods. Also, the inhibitory effect of all endophyte samples in DMC and SMeT were reported by T-test and ANOVA statistical tests.
Results
Isolated Bacterial Endophytes
A total of 11 bacterial endophytes were collected from various parts of the herb including stem, leaf, onion and flower. The results have demonstrated the different effects of the bacterial endophytes isolated from the A. jesdianum on some examined pathogens. Out of 11 endophytes isolated from this plant, 4 bacterial endophytes were gram-positive and 7 gram-negative. Amongst these endophytes, 6 were Cocci, 3 Bacilli and 2 Cocco bacillus. The endophytes with both antimicrobial and antifungal properties belonged to both gram-positive and gram-negative groups with different forms. Most of the bacterial endophytes obtained from the plant were cocci and bacilli. All 11 endophytes isolated from A. jesdianum were grown in different culture media such as Yeast Extract Agar, Peptone Agar, Blood Agar, TSB, LB (Luria Bertani Broth) and also Manitol salt Agar. Colonies of bacterial endophytes in the Peptone Agar medium showed the better growth. Furthermore, the colonies in the YEA medium were weaker than that of the PA medium.
The secretory metabolites of the isolated bacterial endophyte from all parts of the plant (stem, leaf, onion and flower) showed remarkable and favorable antibacterial and antifungal effect on the examined pathogens (Table 1-2). In general secretory metabolites of A. jesdianum’s bacterial endophytes demonstrated the better inhibitory growth effects than endophytes.
Antibacterial Effect of A. jesdianum Against S. aureus (ATCC: 25923)
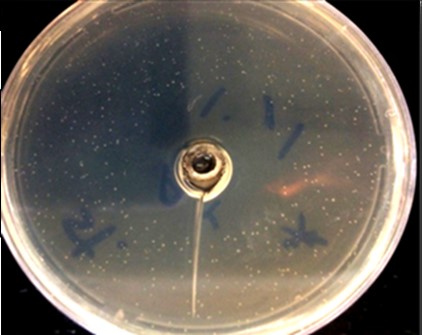
In this study, through the drip method by chloroform (DMC), endophytes of A. jesdianum showed a favorable effect on S. aureus (ATCC: 25923) (Figure 1).
Figure 1. Growth inhibiting effect of A. jesdianum endophyte called "M" on S. aureus (ATCC:25923) by chloroform drip method.
In general the best antibacterial effect of endophytes was on S. aureus (ATCC: 25923). However, the secretory metabolites of most isolated bacterial endophytes showed favorable inhibitory effects on S. aureus (ATCC: 25923) (Figure 2).
Figure 2. Antibacterial effect of A. jesdianum endophyte “S” metabolite on S. aureus
Antibacterial Effect of A. jesdianum Against E. coli (ATCC: 25922)
None of the 11 bacterial endophytes isolated from A. jesdianum were effective in the two methods, per chloroform drip, and evaluating the effect of secreting metabolites on E. coli (ATCC: 25922) (Table 1).
A very weak growth inhibitor zone against E.coli was created by the secretory metabolites of 2 isolated endophytic bacteria (C1 and C2) (Table 2).
Antifungal Effect of A. jesdianum Against C. albicans
Using the chloroform drip method, only the endophytes named “K”, “H” and “Z”, which have been isolated from the flowers and bulbs of A. jesdianum, created a very weak growth inhibition against C. albicans. In other bacterial endophytes, no growth inhibitory zone was observed against C. albicans, however three endophytes created a very small zone (Table 1).
The secretory metabolites of the isolated endophytic bacteria from A. jesdianum showed relatively positive inhibitory effects on C. albicans and only one bacterial endophyte “W” showed no growthless halo (Table 2).
Table 1. Mean diameter (SD) of growth inhibition zone of bacteria (S. aureus & E. coli) and fungus (C. albicans) in millimeters based on bacterial endophytic structure factors in A. jesdianum by bacterial colony drip method using chloroform
| Endophytes | Morphology | Plant section | S. aureus (SD) |
E. coli (SD) |
C. albicans (SD) |
| K | Bacilli G - | Onion | 34.5(1.5)* | 0(0) | 4(2) |
| M | Cocci G - | Leaf | 36.5(3.5) | 0(0) | 0(0) |
| F | Cocco Bacillus G+ | Leaf | 18.5(3.5) | 0(0) | 0(0) |
| C1 | Cocci G+ | Leaf | 5(1) | 0(0) | 0(0) |
| C2 | Cocci G+ | Leaf | 1.5(1.5) | 0(0) | 0(0) |
| S | Cocci G+ | Leaf | 0(0) | 0(0) | 0(0) |
| W | Cocco Bacillus G - | Leaf | 0(0) | 0(0) | 0(0) |
| B1 | Cocci G+ | Stem | 0(0) | 0(0) | 0(0) |
| B2 | Cocci G+ | Stem | 12.5(2.5) | 0(0) | 0(0) |
| H | Bacilli G - | Onion | 15(1) | 0(0) | 2(2) |
| Z | Bacilli G - | Flower | 2(2) | 0(0) | 6(1) |
* The number in parentheses indicates the standard error of mean (SEM).
Table 2. Mean diameter (SD) of growth inhibition zone of bacteria (S. aureus & E.coli) and fungus (C. albicans) influenced by endophytic bacterial secretory metabolites of A. jesdianum
| Endophytes | Morphology | Plant section | S. aureus (SD) | E. coli (SD) | C. albicans (SD) |
|---|---|---|---|---|---|
| K | Bacilli G - | Onion | 27(3)* | 0(0) | 10.5(2.5) |
| M | Cocci G - | Leaf | 21.5(3.5) | 0(0) | 8.5(1.5) |
| F | Cocco Bacillus G+ | Leaf | 0(0) | 0(0) | 13(2) |
| C1 | Cocci G+ | Leaf | 18.5(5.5) | 2(0) | 9(1) |
| C2 | Cocci G+ | Leaf | 21(1) | 3(0) | 12.5(1.5) |
| S | Cocci G+ | Leaf | 27(3) | 0(0) | 11.5(1.5) |
| W | Cocco Bacillus G - | Leaf | 14.5(0.5) | 0(0) | 0(0) |
| B1 | Cocci G+ | Stem | 26.5(3.5) | 0(0) | 11(1) |
| B2 | Cocci G+ | Stem | 0(0) | 0(0) | 14(1) |
| H | Bacilli G - | Onion | 24(2) | 0(0) | 12.5(1.5) |
| Z | Bacilli G - | Flower | 35.5(4.5) | 0(0) | 15(2) |
* The number in parentheses indicates the standard error of mean (SEM).
Antifungal Effect of A. jesdianum Against T. mentagrophytes
Using the chloroform drip method, out of 11 isolated bacterial endophytes, only 4 endophytes named “S”, “Z”, “W”, and “K”, which were isolated from the flowers, onions, and leaves of A. jesdianum, showed antifungal activity against the T. menta-grophytes. The secretory metabolites of the 11 bacterial endophytes isolated from A. jesdianum showed no effect on the T. mentagrophytes growth (Table 3).
Antibiogram Test
The results of antibiogram test was using the disc diffusion method are presented in the diameter of the growth inhibition zone (Table 4).
Statistical Analysis
There was a significance difference between all endophytes’ antimicrobial effect in using drip method by chloroform (DMC) compared to secretory metabolite test (SMeT) in term of inhibitory effect on S. aureus.
Table 3. Antifungal properties of endophyte bacteria against Trichophyton mentagrophytes growth based on chloroform drip method and endophytic bacterial secretory metabolites of Allium jesdianum
| Effect of endophytic structural factors | Effects of endophytic secretory metabolites | Plant section | Morphology | Endophytes |
| Negative | Negative | Stem | Cocci G+ | B1 |
| Negative | Negative | Stem | Cocci G+ | B2 |
| Negative | Negative | Leaf | Cocci G+ | C1 |
| Negative | Negative | Leaf | Cocci G+ | C2 |
| Positive | Negative | Leaf | Cocci G+ | S |
| Positive | Negative | Leaf | Cocco Bacillus G - | W |
| Negative | Negative | Leaf | Cocci G - | M |
| Negative | Negative | Leaf | Cocco Bacillus G+ | F |
| Negative | Negative | Onion | Bacilli G - | H |
| Positive | Negative | Onion | Bacilli G - | K |
| Positive | Negative | Flower | Bacilli G - | Z |
Table 4. Diameter of growthless aura created in antibiogram test against S. aureus & E. coli.
| Antibiotics | S. aureus (ATCC: 25923) |
E. coli (ATCC:25922) |
| Doxycycline | 13 | _ |
| Chloramphenicol | 14 | 24 |
| Cefteriaxone | 0 | 20 |
| Nalidixic acid | 0 | _ |
| Furazolidone | 19 | 19 |
| Ampiciline | 0 | 0 |
| Lyncomycin | 20 | _ |
| Streptomycin | 18 | 14 |
Except for endophytes “S”, a significance difference was reported between all endophytes using the drip method by chloroform compared to secretory metabolite test in term of inhibitory effect on C. albicans.
Amongst all endophytes, “C1” and “C2” showed a significant difference inhibitory effect on E. coli in both methods. The obtained data from the effect of various endophytes on growing of three pathogens in both methods are presented in Table 5.
Table 5. Comparison between the inhibitory effects of Allium jesdianum endophytes on growing of three pathogens in both methods.
| Bacterial Endophyte | S. aureus DMC |
S. aureus SMeT |
P-value | E. coli DMC |
E. coli SMeT |
P-value | C. albicans DMC |
C. albicans SMeT |
P-value |
| B1 | 0.00±0.00 | 26.00±3.60 | 0.006 | 0.00±0.00 | 0.00±0.00 | 1 | 0.00±0.00 | 12.00±2.00 | <0.001 |
| B2 | 12.33±2.51 | 0.00±0.00 | 0.001 | 0.00±0.00 | 0.00±0.00 | 1 | 0.00±0.00 | 12.66±2.08 | 0.009 |
| F | 18.00±3.6 | 0.00±0.00 | 0.013 | 0.00±0.00 | 0.00±0.00 | 1 | 0.00±0.00 | 13.33±2.09 | 0.008 |
| M | 36.00±3.67 | 21.33±3.51 | 0.007 | 0.00±0.00 | 0.00±0.00 | 1 | 0.00±0.00 | 8.66±1.52 | 0.010 |
| H | 15.00±1.00 | 23.66±2.51 | 0.005 | 0.00±0.00 | 0.00±0.00 | 1 | 2.07±1.67 | 11.66±1.08 | 0.004 |
| C1 | 4.66±1.54 | 18.33±5.50 | 0.014 | 0.00±0.00 | 1.00±0.93 | <0.001 | 0.00±0.00 | 8.33±1.50 | 0.001 |
| C2 | 1.33±1.52 | 19.66±2.50 | <0.001 | 0.00±0.00 | 1.57±1.37 | <0.001 | 0.00±0.00 | 13.33±2.05 | 0.008 |
| K | 34.00±1.73 | 27.00±3.00 | 0.025 | 0.00±0.00 | 0.00±0.00 | 1 | 3.66±2.08 | 10.66±2.51 | 0.021 |
| S | 0.00±0.00 | 26.666±3.05 | <0.001 | 0.00±0.00 | 0.00±0.00 | 1 | 0.00±0.00 | 11.66±1.49 | <0.001 |
| W | 0.00±0.00 | 15.33±1.52 | <0.001 | 0.00±0.00 | 0.00±0.00 | 1 | 0.00±0.00 | 0.00±0.00 | 1 |
| Z | 2.00±1.82 | 34.66±4.72 | <0.001 | 0.00±0.00 | 0.00±0.00 | 1 | 6.00±1.41 | 15.00±2.00 | 0.010 |
DMC: Drip method by chloroform
SMeT: Secretory metabolite test
Intra-group Analysis
A significance difference between the “B1”, “S” and “W” with other endophytes in terms of inhibitory effects on S. aureus was reported in DMC method (P<0.05). There was also, significant difference amongst other endophytes including M, K, F, H, B2, C1, Z, and C2 in terms of inhibitory effects on S. aureus by DMC method (Figure 3).
S. aureus significantly showed a difference growing pattern using SMeT (P<0.05). Specifically, a significant difference between the “B2” and “F” endophytes with other endophytes in terms of inhibitory effects on S. aureus was observed (P<0.05).
The inhibitory effect of all endophytes in DMC and SMeT on each of the S. aureus was showed in Figure 3.
The obtained data from inhibitory effect of various endophytes on C. albicans by both groups are expressed in Figure 4. A significant difference between the “W” endophyte with other endophytes was observed in terms of inhibitory effect on C. albicans in DMC method (P<0.05). While, the “W”, “C1”, “M”, “S”, “B1”, “C2” and “F” endophytes showed significant difference respected to inhibitory effect compared to “H”, “Z” and “K” endophytes on C. albicans by SMeT (P<0.05) (Figure 4).
There was no significant difference amongst various endophytes in terms of inhibitory effect on E. coli using SMeT and DMC.
Figure 3. The inhibitory effect of A. jesdianum endophytes on S. aureus in both methods, DMC: drip method by chloroform SMeT: secretory metabolite test; *, #, & characters show the significant difference, P<0.001, P<0.01, and P<0.05, respectively.

Figure 4. The inhibitory effect of A. jesdianum endophytes on C. ablicans in both methods, DMC: drip method by chloroform SMeT: secretory metabolite test, *, #, & characters show the significant difference P<0.001, P<0.01, and P<0.05, respectively.
Discussion
Since this study was designed to investigate the effect of Allium jesdianum’s bacterial endophytes on the certain fungal and bacterial pathogens, the main findings of the present study was the potency and effectiveness of the bacterial endophytes in inhibiting of the aforementioned human pathogens’ growth. Eleven bacterial endophytes were obtained from various sections of A. Jesdianum. In general, endophytes have an improved inhibitory effect on T. mentagrophytes. Secretory metabolites of endophytic bacteria had a positive effect on S. aureus and
C .albicans.
Endophytic bacteria have been widely obtained from a variety of plants that sustain one or more endophytes in 300,000 species on the earth (23). Until now, several studies have been attempted to identify and isolate microbial endophytes from a variety of medicinal plants in which the antifungal and antibacterial properties of endophytes have been demonstrated (24).
The method proposed by the present study for the superficial sterilization of the plant is an effective method for the isolation of endophytes. The process of removing epiphytic microorganisms and rhizo-spheres from the soil, which are brought to the laboratory with plant samples, has been used in several ways over the last few years. Washing the samples by alcohol and sodium hypochlorite is one of the main and constant steps (25,26). To ensure sterilization of the samples surface, the last distilled water solution (used for washing) was cultured and the presence of bacteria was detected. The lack of bacterial growth indicates that the surface steriliza-tion steps were performed correctly. It can be ensured that the bacteria isolated from the plant samples were endophytes. In addition to the high growth of A. jesdianum endophytic bacteria, this paper provides a simple and powerful method of surface sterilization of plant tissues for better separation of endophytes in the plant.
The anti-diabetic and anti-tumor properties of endophytes have been recently demonstrated (27). Sebola et al. (2019) showed the antibacterial activity of extract of bacterial endophytes (28). The existence of endophytics in various parts of plants has also been reported previously (29,30). Vu et al., (2019) have isolated 111 endophytic actinomycetes from roots, stems and leaves of the Cinnamomum cassia Presl, with different antimicrobial activity (30).
In the present study, the endophytes were mainly isolated from the leaves compared to other parts. “M” endophyte (36 ± 3.67) in the chloroform drip method and “Z” endophyte (34.66 ± 4.7) in endophytic secretory metabolite test showed significant effects on inhibition of S. aureus.
There was a significant difference amongst all endophytes against S. aureus indicated by the mean diameter of the growth inhibition zone using both methods(P  0.05).
0.05).
All 11 endophytes isolated from A. jesdianum were grown in Manitol salt Agar, after 24-48 hours, which indicates they have a high tolerance to sodium chloride salt. Microorganisms’ growth inhibitory effect of the plant extracts is caused by some of the phytochemical compounds such as tannins, saponins, terpenoids, alkaloids, flavonoids, phenols and ster-oids, etc. that is extracted by chloroform (31). Allium species contain vital compounds such as carbohyd-rates, flavonoids and saponins. These species possess important biological and pharmacological activities such as antifungal, antibacterial, anti-tumor, anti-inflammatory, anti-thrombotic and hypocholestero-lemia properties (32, 33). The antimicrobial and antifungal properties of endophytes compounds are possible to be extracted by chloroform (34, 35). The antioxidant properties of Allium are related to the flavonoids and phenolic compounds (36).
Amiri (2007) demonstrated that ethanolic extract has stronger antimicrobial effects than those of the essential oil and other extracts of A. jesdianum. The antimicrobial properties of oil and ethanolic extracts are related to their sulfide and terpenoid compounds (37). Studies demonstrated that Allium bakhtiaricum chloroform fraction has a suppressive effect on breast cancer (38). The antioxidative properties of allium species possibly function with organosulfur compo-unds (39). El-Gendy et al. (2018) have carried out studies on the bioactive metabolites from various marine endophysical Streptomyces species against MRSA (40).
The antifungal activity and immunomodulatory effect of A. jesdianum Boiss extracts against C. albicans has been shown (41). According to statistical analyses, “K”, “Z” and “H” endophytes have significant inhibitory effects on C. albicans’s growth by both methods (P  0.05). In the present study, the secretory metabolites of endophytic bacteria isolated from A. jesdianum demonstrated relatively favorable inhibi-tory effects on C. albicans.
0.05). In the present study, the secretory metabolites of endophytic bacteria isolated from A. jesdianum demonstrated relatively favorable inhibi-tory effects on C. albicans.
Of the eleven isolated endophytes, only one endophyte, which was named “W”, showed no effect on the growth of C. albicans. Furthermore, endophytic bacteria had the better antifungal properties against C. albicans compared to T. mentagrophytes. The secretory metabolites of all the isolated bacterial endophytes from A. jesdianum showed no effect on the growth of T. mentagrophytes, however the bac-teria’s body had more favorable inhibitory effect on this fungus compared to secretory metabolites. Secondary compounds such as pigments, plant growth regulator factors and mycotoxins, are among the metabolites produced by these endophytes (42).
The secretory metabolites of A. jesdianum may contain antibacterial, antifungal and other pharma-ceutical compounds. In general, the secretory meta-bolites of bacterial endophytes of A. jesdianum show-ed the stronger inhibitory effect than those of bacte-ria’s body against C. albicans.
In disc diffusion antibiogram (Kirby Bauer) tests against S. aureus (ATCC: 25923), the largest diameter of the no-growth zone was 20 mm which belonged to the Lincomycin antibiotic. Whereas, in the metabolite study, the mean diameter (SD) of the growthless zone created was 35.5 mm which belonged to an endophytic bacterium called “Z”, in the DMC, the standard mean diameters of the growth inhibition zone created by bacterial endophytes known as “M” and “K” were 36.5 mm and 34.5 mm, respectively. Because of the widespread and increasing bacterial and fungal infections resistance to antibiotics, isolation and identification of bacterial endophytes can be a good approach to overcome the resistance of human pathogens against antibiotics (43). The obtained data from the present study indicate that the resulting bacterial endophytes have a better inhibitory effect on gram-positive bacteria. The achievement of this important objective is the use of these endophytic bacteria in the production of medication with less side effects and the better effect against Gram-positive bacteria and pathogenic fungi.
Jiang et al. (2018) investigated the 101 actinobacteria as endophytes in which 31 strains showed positive inhibitory effect on at least one bacterium and 21 strains showed inhibitory activity against at least one "ESKAPE" (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acin-etobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species) resistant pathogens (44). Num-erous bioactive compounds derived from endophytes are used as a major resource in various pharma-ceutical formulations (45). Endophytic bacteria play an important role in both medicine and pharmacy. The bacterial endophytes of A. jesdianum and their properties can be used to produce antimicrobial agents, particularly for pathogens that are resistant to common antibiotics. In addition, they can be applied in agriculture and food industries.
Acknowledgements
Acknowledgements: This work was supported by Shahrekord University (grant numbers: 96GRD1M1-801 and 96PRM2M50457).
Funding
The financial resources of this research were provided by Ms. Habibi Nava
Conflicts of Interest
The author declared no conflict of interest.
Received: 2020/09/18 | Accepted: 2020/12/21 | ePublished: 2021/06/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |