BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1193-en.html


 , Zahra Zamani2
, Zahra Zamani2 
 , Marjan Sabbaghian3
, Marjan Sabbaghian3 

 , Ramezan Ali Khavari-Nejad1
, Ramezan Ali Khavari-Nejad1 
 , Mohammad Arjmand2
, Mohammad Arjmand2 


2- Department of Biochemistry, Pasteur Institute of Iran, Tehran, Iran.
3- Department of Biology, Faculty of Basic Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran. ,
.
Malaria is a preventable, treatable, and life-threatening disease caused by disease-related parasites, which are transmitted to humans by mosquito bites. According to the World Health Organization (WHO) annual report on malaria, in 2018 there were about 228 million cases of malaria and 405,000 deaths due to this disease worldwide (1). According to the official report of the WHO in 2017, 57 cases of native malaria were registered in Iran, which shows a significant decrease compared to 1800 cases in 2010 and 12000 cases in 2000. In addition, no deaths from malaria were reported in Iran in 2017 (2).
In the complex life cycle of Plasmodium parasites, asexual blood stage parasites are responsible for the development of clinical signs and symptoms of the disease, and therefore major antimalarial drugs target this part of the parasite's life cycle and are used to improve the symptoms of the disease. Gametocytes, which are involved in the sexual part of the parasite's life cycle, play no role in causing the symptoms of the disease, but they play a role in transmission of the parasite from the human host to the Anopheles mosquito vector (3).
Drugs that can reduce gametocytogenesis, or can kill gametocytes, called gametocytocides, are very effective in counteracting the spread of malaria but due to lack of proper quantitative high throughput screening assays are still being studied. These transmission-blocking antimalarial drugs can work by targeting the following: 1. Effective and complete killing of adult gametocytes when they form in a human host. 2. Inhibition of gametocyte growth to oocytes and eventually sporozoites in mosquitoes. This requires enough medicine to reach the midgut of mosquito from the blood sample (4).
Gametocyte production occurs through five stages of maturation (I to V), and stage V is the only form that can infect mosquitoes. For P. falciparum, these mature gametocytes appear 12 days after symptoms and circulate for an average of 2.5 to 6.5 days, lasting up to 22 days. Thus, circulating gametocytes can maintain the process of malaria transmission from host to vector after drug treatment, which eliminates the symptoms of the disease (5).
Most currently approved antimalarial drugs, including artemisinin (ART)-based combination therapies (ACT), are effective only against blood stages and early-stage gametocytes up to stage III and possibly stage IV gametocyte maturation. In addition, some drug treatments such as chloroquine (CQ) and sulfadoxine-pyrimethamine induce gametocytogenesis and therefore effectively increase the number of cases of disease transmission and the rate of new infections (6).
Currently, the only antimalarial drug that has effective gametocytocidal activity is primaquine, which acts against gametocytes of all Plasmodium spp. and is the WHO recommended option against P. falciparum gametocytes. Unfortunately, the possibility of using this drug is also limited - due to the possibility of acute hemolytic anemia associated with deficiency of glucose 6-phosphate dehydrogenase (G6PD) enzyme (7, 8). Because of the risks of the primaquine treatment, the new transmission-blocking interventions to achieve the ultimate goal of eradicating malaria are currently receiving considerable attention. Extensive studies are recently underway on the biological characteristics of the transmission stages and the development of in vitro assays focusing on the late-stage gametocyte production, mature gametocytes lethality, and gametocyte-oocyte / sporozoite transmission (3). One of the new tests used for the parasite gametocyte stage is the parasite viability assay using the lactate dehydrogenase enzyme (LDH) (9). Lactate dehydrogenase is the enzyme located at the end of the anaerobic pathway of Embden-Meyerhof glycolysis and plays a critical role in the carbohydrate metabolism of human malaria parasites. LDH has been shown to play an important role in malaria infection and may be a possible drug target for malaria treatment (10). In addition to being a marker of parasite viability, P. falciparum LDH (PfLDH) is also an interesting diagnostic biomarker, as it is highly conserved among all four species known to infect humans (11). However, PfLDH activity in the host's circulation is cleared within 24 hours after successful treatment, resulting in much less false-positive diagnosis due to the continued presence of a biomarker after removal of the infection (12).
To achieve malaria elimination, antimalarial drugs or combination therapies must not only eliminate the asexual stages of the parasite that are responsible for the clinical symptoms of disease, but must also be able to clear the sexual stages of the parasite that maintain the host-to-vector transmission stage. Therefore, the results of such studies can be a way to prepare novel transmission-blocking antimalarial drugs.
Eosin B (EO) is a laboratory dye that has previously been proposed for its anti-parasitic ability through molecular docking methods and its antimalarial effect has been studied in in vitro and rodent malaria models (13-16). In the present study, after culturing P. falciparum gametocytes and carefully examining the blood and sex stages of the parasite during the culture period, the EO compound was investigated as a new anti-gametocyte agent and the results of treatment of asexual and sexual stages of the parasite by eosin B using LDH test eosin B has been compared.
In vitro Culture of P. falciparum Asexual Stage Parasites
Culture of P. falciparum strain 3D7 was performed at 37°C in human type O+ RBCs at 5% haematocrit. Culture medium contained complete medium including RPMI 1640 medium (Sigma-Aldrich), 25 mM HEPES (Sigma-Aldrich), 0.2% D-glucose (Sigma-Aldrich), hypoxanthine 200 μM (Sigma-Aldrich), 0.2% Sodium hydrogen carbonate (Sigma-Aldrich), gentamicin (Invitrogen) 40 mg. L-1, 0.5% albumex (Invitrogen) and 10% (vol/vol) O+ human serum. The parasite was cultured in an atmosphere containing 90% N2, 5% O2 and 5% CO2 (17, 18).
The culture medium was daily gassed and replaced with a new medium (heated to 37°C). Parasite proliferation on each day of the culture period was microscopically examined by taking Giemsa-stained thin blood smear. To synchronize asexual culture, 5% D-sorbitol was used to prepare the parasite in the early trophozoite stage (ring) (19).
Induction of Gametocytogenesis and Gametocyte Culture
Asexual parasites were cultured to increase the parasitemia to 6-10%. Then the parasitemia was descended to 0.5% (in 6% hematocrit). Cultures were maintained in an atmosphere containing 90% N2, 5% O2 and 5% CO2 without shaking. The cultures were also maintained at 37°C during daily media change. After 72 hours, the hematocrit was reduced to 3% (day 0). Gametocytogenesis was subsequently monitored by daily microscopic examination of the culture medium. On days 6 to 9, asexual forms were removed by treatment with 100 µg. mL-1 heparin (Sigma-Aldrich). Gametocytes were monitored daily by examining Giemsa-stained thin blood smear until they reached stage V and were prepared for further testing (20).
Quality Control of Functionally Viable Mature Stage V Gametocyte Production and Male Gamete Exflagellation
After production of stage V gametocytes, during daily change of culture medium, the precipitated blood cells were resuspended. 200 μL was taken from the culture medium and quickly transferred to a 1.5 ml tube prewarmed to 37°C. After treating the sample with 50 μM xanthurenic acid in an exflagellation buffer (RPMI 1640 with 25 mM HEPES, 0.2% sodium bicarbonate, pH 8), the culture medium was placed in a microcentrifuge at 2000 g for 30 s at room temperature. Then a thin blood smear was prepared from it and stained with Giemsa and then a light microscope was used to control the quality of exflagellation (21).
Eosin B Test on the P. falciparum Ring Stage
In a 96-well plate, 20 μL of culture containing added asexual parasites was added to all rows except one row belonging to the control group. All samples were repeated in triplicate and placed in a CO2 incubator for 48 hours.
Eosin B Test on Plasmodium Falciparum Gametocyte
In a 96-well plate, 20 μL of gametocyte-containing culture was added to all rows except one row belonging to the control group. All samples were repeated triplicate and placed in a CO2 incubator for 42 hours.
Parasitemia and Gametocytemia Evaluation by P. falciparum Lactate Dehydrogenase (PfLDH) Assay
Parasitemia evaluation by lactate dehydrogenase assay is a high-throughput screening assay for antimalarial agents. For this purpose, 100 μL of Malstat reagent (1.57 g Tris HCl, 2 g L-lactic acid, 200 μL Triton X-100 in 85 ml double-distilled water, 66 mg 3-acetylpyridine adenine dinucleotide (APAD), pH 9.1) was added to the 96 –well microplate. Then 20 microliters of infected or uninfected red blood cells were added. The plate was incubated at room temperature and gently shaken for a few minutes to dissolve the red blood cells. During incubation, equal volumes of Nitro Blue Tetrazolium (NBT) and Phenazine Ethosulphate (PES) were mixed away from light and 20 μL of the mixture was added to the wells. The plate was placed away from light. After 30 to 60 minutes, the color change was controlled so that the color tended to dark purple. The plate was read using a BioTek PowerWave XS Microplate Reader at 650 nm. Uninfected red blood cells were used as a reference. Viability percentage was calculated from the following formula (9, 22, 23)
Viability% = 100 x (ODtreated sample – μc-) / (μc+ - μc-μc+ = means (μ) of OD control gametocytes (c+ )
μc- = means (μ) of OD blank uninfected RBCs (c-)
Statistical analysis: In this study, the tests were repeated 3 times and the results were analyzed by one-way ANOVA at a significance level of p-value <0.05 using GraphPad Prism Version 7.05 software.
Culture of Asexual Parasites
After adjusting the culture conditions and using the appropriate protocol, asexual parasites were cultured. Figure 1 shows the number of parasites during the culture period
Figure 1. Asexual blood stages parasitemia during gametocyte culture.
Figure 2. Different stages of P. falciparum 3D7 during the gametocyte culture period.
As can be seen in the diagram, the number of parasites gradually increases from the beginning of the culture period and reaches its maximum on day 4. Then their number gradually decreased and the parasitemia of asexual sex blood parasites was diminished.
Gametocyte Culture
Figure 2 shows which stages of the parasite are found in the culture medium on different days of the culture period.
The diagram shows the asexual stages around the first to fifth days and the early (I, II and III) and late stage (IV and V) gametocytes were observed in the culture on the second to eighth and seventh to twelfth days, respectively.
Changes in the Number of Gametocytes During the Culture Period
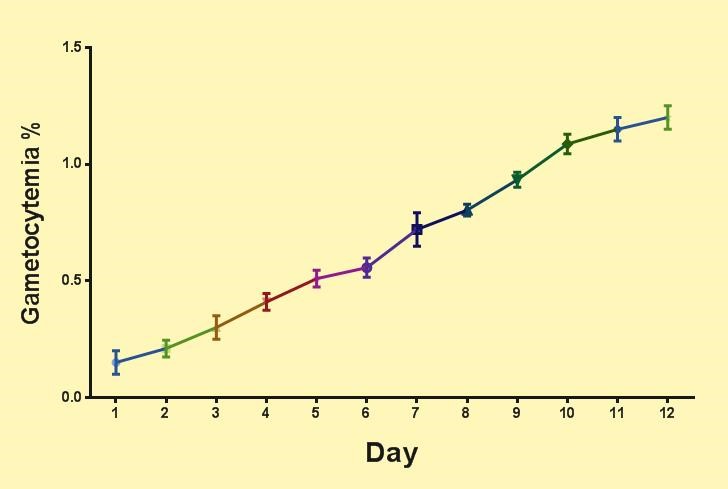
As can be seen in Figure 3, the number of gametocytes gradually increases during the culture period and reaches its maximum on day 12 at the same time as the gametocyte matures (stage V). In this condition, the gametocytes are capable of infecting the vector and are ready to perform an anti-gametocyte reagent test.
The Effect of Eosin B on Asexual Stages of P. falciparum
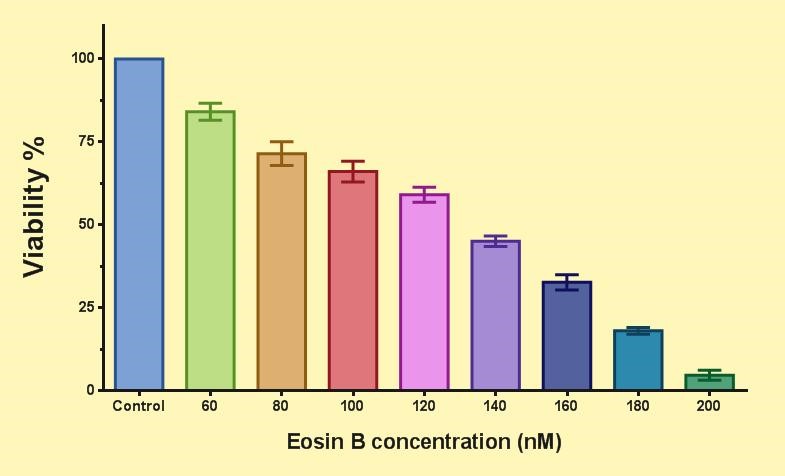
Figure 4 shows the effect of EO on the asexual stages of P. falciparum. Compared to the control group, the viability decreased for each test group by increasing the concentration of EO. The IC50 of EO for the asexual stages of the parasite is 133 nM.
Figure 5 compares the effects of EO and the control drugs CQ and ART on the parasite ring of P. falciparum 3D7. The IC50 level of EO is higher than standard control drugs and is 133 nM. IC50 values for CQ and ART were 6.8 and 7.6 nM, respectively.
Figure 3. Percentage of gametocytemia during gametocyte culture.
Figure 4. The effect of eosin B on the ring of P. falciparum 3D7 parasite. The viability (%) compared to the control group is shown for each test group.
(Means ± SEM (n=5 & P<0.05), One Way-ANOVA test with GraphPad Prism version (7.05)
Figure 5. Comparison of the effect of eosin B (EO) and control drugs chloroquine (CQ) and artemisinin (ART) on P. falciparum 3D7 parasite ring. IC50 values for EO, CQ and ART are shown below each group. (Means ± SEM (n=5 & P<0.05), One Way-ANOVA test with GraphPad Prism version 7.05)
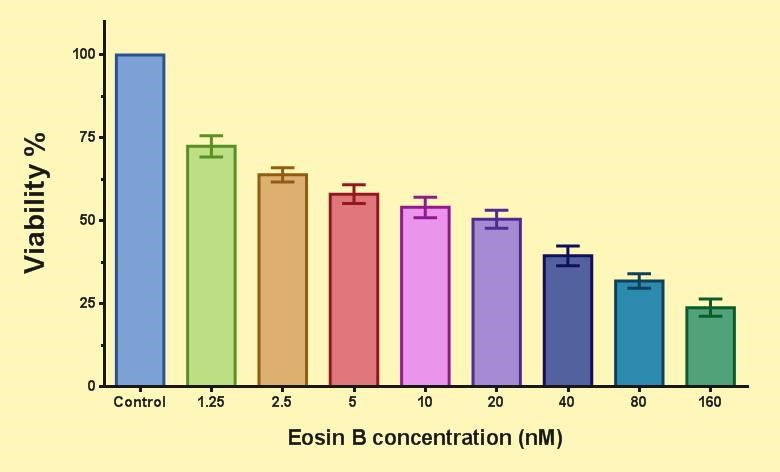
Figure 6. Effect of eosin B on P. falciparum 3D7 gametocyte.(Means ± SEM (n=5 & P<0.05), One Way-ANOVA test with Graph Pad prism version 7.05)
The Effect of Eosin B on P. falciparum Gametocytes
Figure 6 shows the effect of EO on P. falciparum parasite gametocytes. Compared to the control group, the viability decreased for each test group by increasing the concentration of EO. The IC50 of EO for the parasite gametocyte is 23 nM.
Figure 7 compares the effects of EO and the control drugs CQ and ART on P. falciparum 3D7 gametocytes. The IC50 value of EO is lower than 23 nM compared to standard control drugs. IC50 values for CQ and ART were 41 and 85 nM, respectively. Contrary to the results of the EO test on the blood stages of the parasite, it was observed that EO inhibited the gametocyte growth at a lower dose than standard drugs, indicating a potent inhibitory effect of EO on the P. falciparum 3D7 gametocyte.
Figure 7. Comparison of the effect of eosin B (EO) and chloroquine (CQ) and artemisinin (ART) control drugs on P. falciparum 3D7 parasite gametocytes. IC50 values for EO, CQ and ART are shown below each group.(Means ± SEM (n=5 & P<0.05), One Way-ANOVA test with Graph Pad prism version 7.05).
Sustained control of malaria is achieved if, in addition to using therapeutic strategies that target the asexual forms that cause the symptoms of malaria, the sexual forms of the parasite, which are the same as gametocytes, are also targeted by drugs that control the parasite transmission stage (24). In the present study, by an improved protocol of using heparin, induction of gametocytogenesis and production of parasite sex cells was performed to test a new compound in the treatment of P. falciparum strain 3D7. It should also be noted that the control of cultured gametocytes should be done carefully so that at least 90% of gametocytes have reached full IV-V growth stage in order to test the drug composition (25). Also, direct comparison of data from studies of drug discovery against gametocytes is difficult due to several factors, including the following: 1- Parasite strain used; 2- Protocol used to induce gametocytogenesis; 3- Combination of culture medium Used; 4- Gametocyte isolation protocols; 5- Developmental stage of gametocytes in the study; 6- Principles of assays used in the experiment; 7- Presence or absence of erythrocytes; 8- Number of gametocytes in each test well; 10- Concentration of tested compounds; 11- Duration of drug administration; 12- How to express the obtained data such as inhibition percentage in a certain concentration or IC50 alone, etc. (14).
EO has shown a significant inhibitory effect on Toxoplasma gondii and the blood stage of P. falciparum. IC50 values for EO in T. gondii and P. falciparum asexual blood stages were 180 μM and 124 nM, respectively (13, 14). Since the IC50 of EO for P. falciparum gametocytes is 23 nM, EO can inhibit gametocytes more severely than asexual blood parasites. Due to the fact that many antimalarial drugs do not have much ability to eliminate the sexual stage of the parasite and even some (such as CQ) induce gametocyte production and increase the number of gametocytes (26), the significant effect of EO on gametocytes and its anti-gammocyte effect in vitro can be considered for the preparation of drugs that block human-to-mosquito transmission.
Methylene blue, which is a dye used in the laboratory, has an inhibitory effect against all stages of P. falciparum (4) and has been proposed as a strong inhibitor of the transition from host to vector (27). Also, its significant effect on gametocytes of this parasite through morphological deformation of gametocytes has been recently reported (28). The IC50 value of methylene blue for P. falciparum gametocyte in vitro is 12.49 nM (24). The IC50 value for EO is close to this combination, indicating the high efficacy of EO against the sexual stage of the parasite.
Compared to the main antimalarial drugs, it can be mentioned that ART, which is an important antimalarial drug, especially in combination therapies, as well as cases of severe and drug-resistant malaria, cannot directly inhibit gametocytes in the patient's body, but it reduces the gametocytemia indirectly by eliminating the blood stages of the parasite (6). The effect of Artesunate, a derivative of ART, on P. falciparum gametocytes has been previously reported in vitro with an IC50 of 102.3 nM (24). Also, primaquine, which is the only drug used clinically to remove gametocytes, has a much higher IC50 in vitro than EO (IC50 15 μM). Unfortunately, the use of this drug is also limited due to the possibility of hemolytic anemia in people with deficiency in glucose-6-phosphate dehydrogenase (7). Other drugs used clinically against malaria blood stages include quinine and mefloquine, each of which has a higher IC50 than eosin with an IC50 of 50 nM (29). Hydroxychloroquine is another derivative of the important antimalarial drug (CQ) in vitro with an IC50 of 22.78 nM against gametocytes has shown its inhibitory effect (24), which is very close to IC50 EO. Therefore, the efficacy of EO against the sexual stage of P. falciparum is close to or higher than some of the important known antimalarial drugs. Therefore, due to the possibility of using EO as an oral drug and its strong anti-gametocytic effect compared to some conventional drugs, this combination can be considered as a suitable candidate for use as a drug that blocks the transmission from host to vector.
Given the significant effect of EO on the number of gametocytes and also the comparison of its effect on the blood stage of P. falciparum in vitro, this combination will probably be able to effectively control the transmission from human host to mosquito vector. This effect can be tested by infecting Anopheles mosquitoes, which can be considered in future research using the standard Membrane-Feeding Assay test, which is the gold standard test for blocking transmission to be examined (30). Given the lack of known pharmacological agents that block human-to-vector transmission, identifying the EO compound as an antigamocyte agent could be important for future research.
None.
The authors declared no conflict of interest.
Received: 2020/08/1 | Accepted: 2020/12/27 | ePublished: 2021/04/9
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |