BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1090-en.html
2- Assistant professor of Pharmaceutics Research Center, Kerman University of Medical Sciences, Kerman, Iran ,
3- Graduate of General Pharmacy, Student research committee, Kerman university of medical sciences, Kerman, Iran
4- Student of General Pharmacy, Student research committee, Kerman university of medical sciences, Kerman, Iran
.
A biological system can shrink to an infinite size and still maintain its former performance and even add new applications in fact, there are many small cells that are known to be very active in spite of their small dimensions, producing various materials and doing various tasks on their own. This philosophy is one of the starting points for inspiration in nanoscience (1-3). In the recent years, nanotechnology and nanotechnology have begun to make dramatic changes in various sciences, especially medical sciences. It can be widely said that the science and engineering of nanotechnology is defined as the design, fabrication, characterization and application of materials and tools that are particles ranging from a few nanometers to hundreds of nanometers in size (4-6). The overall definition of nanoparticles refers to particles in the range of 1 to 100 nm (7). These particles are designed to exhibit new, specially controlled properties of their raw material in the usual size, which results from precise control over their fabrication process.
One of the most important issues in drug delivery systems and the science of pharmaceutics is improving drug targeting for specific cells and reducing drug accumulation in cells, as high amounts are not necessary for a good function and might be toxic as well (8,9). This is often because the size of commonly used drug molecules is ten times larger than the size of a red blood cell and so the drug penetrates the cells far less than expected and as a result, we will have to increase the dosage or frequency of use to achieve our goals, which can lead to more toxicity and unintended side effects (10,11). Nowadays, by the use of nanomedicine, targeted drug delivery, reduced toxicity and consequently increased efficacy of antimicrobial drugs at lower concentrations have been achieved (10,13,14). On the other hand, in the treatment of infectious diseases, bacterial resistance to newer antibiotics has always been important, and bacterial resistance will lead to increased doses of antibiotics, increased medication to antimicrobial regimens, increased hospitalization and finally, the mortality rate of hospitalized patients will increase (15,16). Treatment of bacterial infections has been a matter of concern in the past until now (17,18). The use of nanotechnology by numerous approaches has so far been identified as one of the most important ways of overcoming bacterial resistance (19-21, 32). Among the things that can be described as a new generation of antibacterial compounds are nanoparticles containing biopolymers such as chitosan (22), nanoparticles containing metals such as Au (23), Ag (24), Mg (25), Cd (26), Bi and Cu (27). Multiple antimicrobial agents are drug bound to nanoparticles (15,16). The necessity of this research is to create nanostructures based on bio polymeric structure with calcium oxide nanoparticles as one of the cheapest nanoparticles for investigation of antimicrobial properties. Also, the use of chemical imaging and microwave radiation as environmentally friendly and cost-effective methods is one of the unique features of this research work. The aim of this study was to synthesize and optimize particle size of calcium oxide nanoparticles loaded with 1%, 0.5% and 0.25 wt% in poly lactic acid with 1%, 0.75% and 0.5 wt% percentage by chemical mimicry and using microwave waves and on 3 gram-positive bacteria evaluation of minimum growth inhibition concentration (MIC) in bacterial strains Micrococcus luteus, Bacillus subtilis, Staphylococcus aureus and 4 gram-negative bacteria Escherichia coli, Klebsiella pneumonia, Serratia marcescens, Pseudomonas aeruginosa.
Synthesis of Cao/PLA nanocomposites
For preparation of PLA/CaO nanocomposites, the dry powder of the nanoparticles synthesized from the preceding steps were blended together as follows.
Sediments from calcium oxide weighing 0.25 grams and sediments from polyolactic acid weighing 0.5 grams, sediments from calcium oxide weighing 0.5 grams and sediments from polyolactic acid weighing 0.75 grams and sediments from calcium oxide weighing 1 g and sediments from polyolactic acid weighing 1 g were mixed and the synthesized nanocomposites were named A1, A2 and A3, respectively. The precipitate mixture was refluxed with 10 ml of the 1: 2 ratios of dimethylformamide (DMF) and water for 30 min at 50 ° C with magnetic stirrer. The mixture was placed in a microwave oven at 300 W for 15 minutes with 1: 2 on-off cycles and the final precipitate was collected from the filter paper dry. Table 1 shows in vitro conditions for making calcium-containing polyelactic acid nanoparticles.
| Temp (◦C) | pH | DMF: H2O (ml) | CaO (g) | PLA (g) | Nanoparticle | Sample |
| 50 | 8-9 | 20 | 0.25 | 0.5 | A1 | 1 |
| 50 | 8-9 | 20 | 0.5 | 0.75 | A2 | 2 |
| 50 | 8-9 | 20 | 1 | 1 | A3 | 3 |
Preparation of Half McFarland Solution
First, 0.5 mL of two hydrated barium chloride was prepared at a concentration of 1.175 wt.% In 1% sulfuric acid. The constant stirring turned out to be a one-stop suspension. The solution density was measured using optical absorption measurement in spectrophotometer with 1 cm optical path length equal to 1.5. The 5 ml solution was poured into coiled tubes the same size as the bacterial suspension tubes and kept in space. The solution was examined before each use for the presence of large particles with the naked eye and then stirred vigorously to create a uniform opacity. Suspension that is similar to the half-McFarland solution for opacity is 1.5 × 108 microorganisms.
Preparation of Muller Hinton agar and Muller Hinton broth culture medium
The 7.6 g of powder was mixed with 200 ml distilled water and then dissolved by heat and continued heating until the solution was clear. It was then removed by pipette, 18 ml of this medium, poured into large reflux tubes and sterilized by autoclaving at 121°C and a pressure of 15 pounds per square inch for 15 minutes. After sterilization, the medium was synthesized with two millimeters of the mixture and the molar Hinton broth medium prepared in various dilutions. It was then removed by pipette, 18 ml of this medium, poured into large reflux tubes and sterilized by autoclaving at 121°C and a pressure of 15 pounds per square inch for 15 minutes. After sterilization, the medium was synthesized with two millimeters of the mixture and the molar Hinton broth medium prepared in various dilutions. To prepare this medium, the culture was poured 1/2 g of the powder in 100 ml of distilled water and stirred until the powder was completely dissolved. Then, two millimeters of the prepared medium was poured into small flask tubes and sterilized by autoclaving at 121°C for 15 minutes at a pressure of 15 pounds per square inch. Mulberry Hinton agar medium was sterilized in autoclave, poured into 18 ml large test tubes. The dilutions of the 2 ml sample were mixed with a well of melt medium and transferred to a plate. Thus, the final dilutions in the plates were 0.5, 1, 2, 4, 8, 16, 32 and 64 μg/mL. Positive and negative control plates were also prepared on the back of the plates before labeling clearly for the concentration of antimicrobial agent and the culture location of each microbe. Bacteria that were cultured 24 h in the remaining 7 tubes containing 2 mL of Müller Hinton broth were microbial suspensions similar to 0.5 McFarland's solution in opium. For this purpose, the loops were sterilized each time by the flame and after that the temperature was lowered to the point that it did not harm the living bacteria, the colonies were removed and dispersed well in a liquid fist medium.
Dc= Kλ/β.cosθ Equation 1
Where θ is the X-ray diffraction angle, Kλ denotes the wavelength of the beam at a constant whose value is 0.9. And the number in dc estimates the diameter of calcium-containing polyelectric acid crystalline nanostructures at about 150 nm.
Table 2. Results of standard bacterial growth at 8 different concentrations of A1 nanoparticles
| 0.5 µg/ml | 1 µg/ml | 2 µg/ml | 4 µg/ml | 8 µg/ml | 16 µg/ml | 32 µg/ml | 64 µg/ml | Concentration Bacteria |
| + | + | - | - | - | - | - | - | E.coli |
| + | + | + | - | - | - | - | - | K. pneumoniae |
| + | + | + | + | - | - | - | - | S. marcescens |
| + | + | + | + | - | - | - | - | P. aeruginosa |
| + | + | + | - | - | - | - | - | S .aureus |
| + | - | - | - | - | - | - | - | M. luteus |
| + | + | + | - | - | - | - | - | B. subtilis |
| + | + | + | + | + | + | + | + | Control + |
| - | - | - | - | - | - | - | - | Control - |
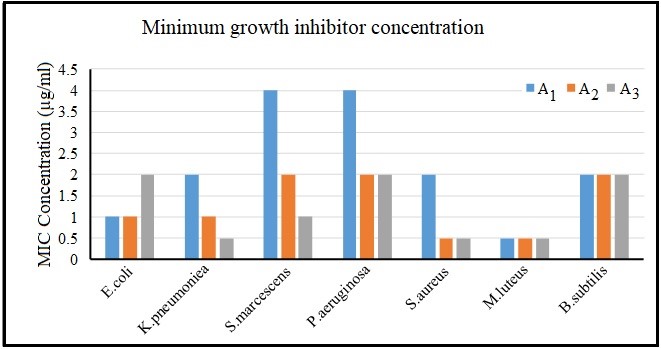
Figure 5. Comparison of growth inhibitory concentration in all three nanocomposites
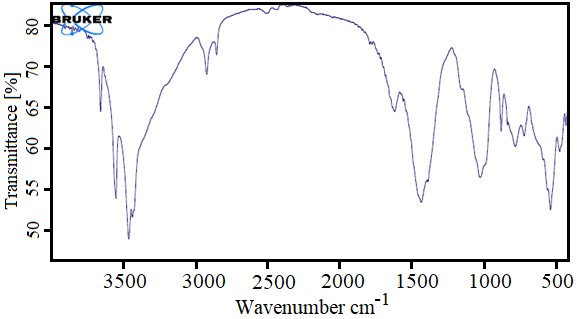
In recent years, the study on the antimicrobial properties of metal oxide has shown promising results. In this study, the optimum nanostructures were selected by particle size and morphology by structural optimization and phase determination. As can be seen in the light diffraction graph of the CaO/PLA crystal nanostructures, the peak has become ring-shaped, confirming the existence of a normal and uniform particle size distribution, which is in clear agreement with the SEM images. The best morphology and uniformity of particle size in A1 nanostructures can also be observed through scanning electron microscopy. And this sample is selected as the optimal sample. Investigation of infrared spectroscopic spectra also shows the loading of calcium oxide nanoparticles in polymeric structures. In line with these studies, it has been observed that biopolymeric polymeric nanostructures containing calcium oxide made by chemical and microwave and hydrothermal synthesis have synergistic effects on Escherichia coli strains, Lactobacillus plantarum, Staphylococcus aureus and Staphylococcus aureus. And they have good antimicrobial properties. Ciprofloxacin as a modern antibiotic is effective against most gram-positive and gram-negative microorganisms and is a good positive test for this test. Pseudomonas aeruginosa 0.5 μg/mL ≤ MIC < 1μg/mL, Streptococcus pyogenes 0.5 μg/mL IC MIC < 1μg/mL, Serratia and Klebsiella 0.25 MIC ≤, Staphylococcus epidermidis 1 μg/mL ≤ MIC <2 μg/mL, Luteus is 8 μg/mL <MIC ≤ 16μg/mL, bacillus MIC> 64 μg/mL, which has fewer positive effects on gram-positive bacteria than the results obtained in this study. In 2014, a study on the antimicrobial properties of Copaiba oil showed that pyrrolidone and polylactic acid increased the amount of oil released from the polymer substrate and compared to our work, the results of this article on bacterial strains have received a weaker antibacterial response (28). In 2007, it was observed that a composite made of pectin polymer and polylactic acid produced a heterogeneous biphasic structure that could be observed by electron microscopy and was able to inhibit the growth of Lactobacillus plantarum and concluded that it can be suitable for the packaging industry (29). In addition, another study in 2018 found that the combination of cinnamon, silver nanoparticles, cobalt and antimicrobial agents such as ciprofloxacin increased the antimicrobial effects, therefore, the applications of this polymer in wound healing and drug release can be of more interest (30). Previously used in a study of polycrystalline acid / silicon / calcium carbonate membrane composite containing mercapto groups (PSC-SH) to investigate the antibacterial and stimulating properties of osteoblast activity. This compound owes its properties mainly to the mercapto group, but this study has also shown that polylactic acid polymer can be a significant substrate for material release (31). In the present study, we tried to compare the microbial effects by combining different concentrations of calcium oxide metal nanoparticles in the polymeric lactic acid as a substrate. As the MIC results show, all concentrations had inhibitory effects on the 7 bacterial strains. However, this inhibition did not follow a fixed pattern.
In one study, three different concentrations of calcium oxide nanoparticles were synthesized by microwave and combined with three different concentrations of polymer nanostructures using aqueous solvent-aqueous (hydrothermal) method with specific ratios. Structural and morphological identification were performed with SEM and XRD analysis. All of the synthesized nanoparticles were nanometer sized, but differed in dispersion and surface properties and the particle size distribution followed the normal distribution. The nanoparticle diameter measurements were confirmed by dynamic scattering of light by electron microscopy images. At all concentrations, inhibitory effects of growth on the tested bacteria were observed. There was no difference in the effect of nanocomposite on gram positive and negative bacteria in the tests. For Gram-positive Bacillus subtilis bacteria the MIC range remained constant with increasing percentage of calcium oxide and observed no effect of increasing antimicrobial concentration. Also, for gram-negative bacteria Escherichia coli, the antimicrobial effect decreased with increasing percentage of calcium oxide. It seems that this compound can be studied for wound healing formulations.
Bio-lactic acid / calcium oxide nanostructures with nanoscale sizes are highly capable of destroying nosocomial microbes and can be used as a highly effective synthetic antibiotic in the pharmaceutical industry.
We would like to thank Kerman University of Medical Sciences, Pharmaceutics Research Center as well as the Student Research Committee of Kerman University for their contribution to this research project.
Authors declared no conflict of interests.
Received: 2020/03/29 | Accepted: 2020/06/14 | ePublished: 2020/05/12
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |