BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1079-en.html

 , Fereshteh Eftekhar2
, Fereshteh Eftekhar2 
 , Fereshteh Shahcheraghi3
, Fereshteh Shahcheraghi3 
 , Mojtaba Noofeli4
, Mojtaba Noofeli4 
 , Seyed Reza Banihashemi5
, Seyed Reza Banihashemi5 

2- Department of Microbiology and Microbial Biotechnology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran ,
3- Department of Bacteriology, Pasteur Institute of Iran, Tehran, Iran
4- Razi Vaccine and Serum Research Institute, Agricultural research, Education and Extension Organization (AREEO), Karaj, Iran
5- Department of Immunology, Razi Vaccine and Serum Research Institute, Agricultural research, Education and Extention organization(AREEO), Karaj, Iran
.
Pertussis is an acute respiratory illness that is highly contagious, especially in infants and children, and is caused by the gram-negative bacterium Bordetella pertussis (1). Pertussis is a reemergence disease and one of the ten most common causes of death from infectious diseases worldwide. Before vaccination was introduced, it was the main cause of infant death in the world. The main source of illness for the infant is family members, and transmission is often directly from one person to another (2).
The first generation of vaccines against the disease, the whole-cell pertussis vaccine (wP), was introduced in 1940. This vaccine uses the whole bacterium which has been killed by heat so that with widespread vaccination in the world, the number of cases decreased rapidly. Although, the vaccine was associated with side effects such as fever and local reaction at the injection site and encephalopathy, due to the high content of lipopolysaccharide, which is highly immunogenic (3). Due to the side effects of this vaccine, the second generation of the acellular pertussis vaccine (aP) was introduced and replaced in developed countries. aP vaccines contain inactivated pertussis toxin, along with recombinant or pure bacterial antigens of B. pertussis (4, 5).
wP provides immunity against a wide range of antigens, while aP creates immunity against a small number of antigens used in vaccine formulations. As a result, aP has low immune-stimulating activity and does not inhibit colonization in the respiratory tract so immunized individuals can be involved in the transmission of the disease in the population (6, 5).
In recent years, the re-emergence of pertussis has been observed in many countries with high vaccinated populations, and there has been a significant increase in the number of reported cases so that despite high rates of vaccination the disease is not yet controllable. Pertussis is an endemic disease, as well as the most common vaccine-preventable disease reported in industrialized countries (8, 7). In Iran, wP vaccines have been produced and used based on standard strain for many years, but despite high vaccination coverage, the number of patients with the disease has increased in recent years (9).
The resurgence of pertussis is associated with a variety of factors, including reduced immunity over time, gene mutations in bacterial antigens, and polymorphism in these genes, resulting in differences between the circulating strains and strains used in vaccine production was noted (11, 10). According to the mentioned points, due to the control of the disease, a new generation of vaccines is needed on which genetic polymorphism is not effective.
Outer membrane vesicles (OMVs) of B. pertussis contain outer membrane proteins (OMPs) and lipopolysaccharide (LPS) that have adjuvant properties and stimulate innate immune responses as well (12, 13). Unlike aP vaccines, these OMVs are easily absorbed by mammalian cells due to their nanoparticle properties and are harvested by antigen-presenting cells (APC) (14, 15). For this reason, to control pertussis disease, OMVs vaccine obtains from vaccinal strain are considered suitable candidates for the vaccine. Therefore, the extraction of these vesicles is of particular importance. To date, various methods have been proposed to stimulate the production and extraction of vesicles. The most important method is based on the use of serial ultracentrifugation (16). But lack of easy access of research and academic centers to ultracentrifuge in our country Iran is an important barrier for the extraction of OMVs. Therefore, the aim of this study was to provide a suitable and high-yield method for OMVs extraction of B. pertussis and to investigate the presence of virulence factors in it.
Strain and Culture
B. pertussis BP134 (vaccinal strain) provided by the Razi vaccine and serum research institute was used in this study. The bacterium was cultured on the Bordet-Gengou agar medium.
Isolation of OMVs
Massive bacterial culture was performed to isolate OMVs on the modified Stainer Scholte (MSS) liquid culture medium (17). Colonies grown on Bordet-Gengou medium were transferred to 50 cc MSS liquid medium under sterile conditions and incubated at 35°C and 150 rpm until bacterial growth reached the end of the logarithmic phase, turbidity equal to 0.7 to 1 at a wavelength of 600 nm (OD600 = 0.7-1), 10 cc of which was inoculated to 500 cc of the new MSS medium. It was shown as OD600 = 0.05. This means that this turbidity was considered the initial turbidity for bacterial growth, then incubated at 35°C and 150 rpm. Taking into account the growth curve of the bacterium and considering that the best time to stop bacterial growth and start the OMV extraction process is at the end of the logarithmic phase and the beginning of the stationary phase, it seems that the best time to harvest a cell mass when the turbidity is 0.7 to 1 at a wavelength of 600 nm.
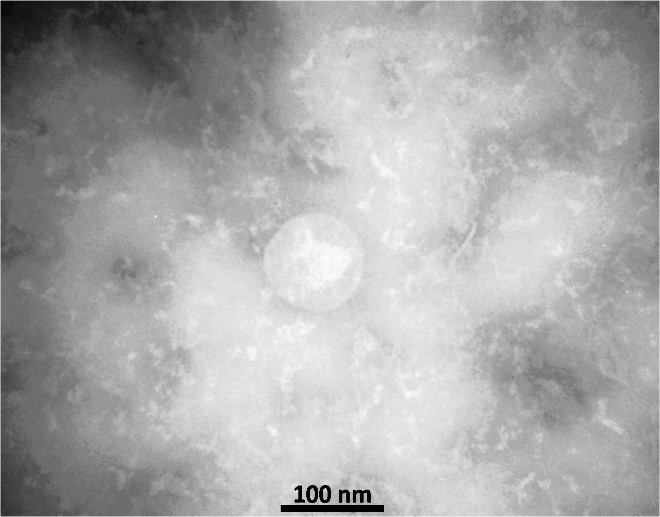
By centrifuging the culture medium containing bacteria at 8000 g and at 4°C for 30 minutes, bacterial pellets were isolated and washed twice with phosphate buffer (PBS). In the next step, the pellets were completely dissolved in the Tris-EDTA buffer (TE; 8 ml per gram of sediment) to create a uniform suspension. The suspension was then kept at room temperature for 30 min and sonicated for 10 min on ice. The suspension was centrifuged at 10000 g and 4°C for 20 min. The pellets were washed with TE buffer and the supernatant was centrifuged at 60000 g C for 2 hours at 4°C. The pellets dissolved in the buffer contained Tris (0.1 mol), EDTA (10 mM) and DOC (5 g/L) to obtain a uniform suspension, then the suspension was centrifuged at 60000 g at 4°C for 2 h. The supernatant was poured into a new microtube and the TE buffer was added to it and centrifuged again at 60,000 g and 4°C for one hour. Then, the obtained pellets were dissolved in 5 ml of 3% sucrose and passed through the 0.22 µm filter. The filtered sample (containing OMV) was inactivated by heating in 56°C water bath. To study sterility, the extracted vesicles were cultured in the blood Agar. OMV extraction was performed four times with this method, and each time, the size and spatial shape of the vesicles extracted with the TEM electron microscope were examined (12, 18). For this purpose, a carbon formvar grade was first prepared and negatively stained with potassium phosphotungstate. The grids were examined by Zeiss EM10C electron microscope (12).
Measure the Amount of Vsicles Protein
Bradford protein testing is a common method for measuring the concentration of proteins. OMV protein levels were measured according to this method (19).
SDS-PAGE Electrophoresis and Western Blotting
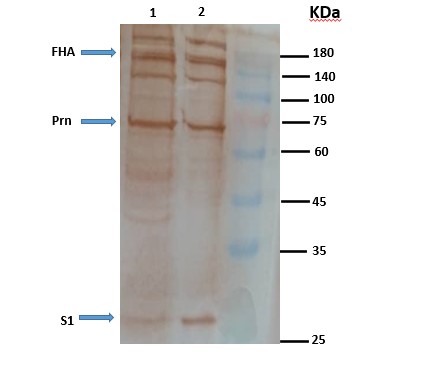
The protein profile of pertussis toxin, pertactin, and filamentous hemagglutinin proteins in OMVs extracted from vaccinal strain was evaluated using the SDS-PAGE method on 12% polyacrylamide gel (20). The coloring of the obtained protein bands was performed by Coomassie blue method and the final confirmation of PTX, PRN, FHA protein expression was prepared by the western blotting method using monoclonal antibodies (NIBSC No. 97/572, 97/558 and 97/564, respectively). NIBSC was performed using SMOBIO (PM2600) protein marker (12, 21).
Pyogeny Test in Rabbits
The pyrogeny testing of the extracted sample was performed according to the Pharmacopoeia method. To a group of five white rabbits produced by the Razi vaccine and serum research Institute, 3 mg/mL of OMVs were injected intravenously per kilogram of animal weight, and the same amount of PBS was injected into the control group. Then the rabbit's body temperature was measured every hour, until the third hour after the injection (22).
Abnormal Toxicity Test
The Abnormal Toxicity Test was performed to determine the harmlessness of the consumption of OMVs extracted in rabbits. A group of five white rabbits weighing 1.5 to 2 kg provided by Razi Vaccine and Serum Institute were injected intramuscularly with 3 mg/ml of OMVs per kilogram of body weight. The rabbits were then kept in normal conditions for seven days. Temperatures were maintained in the range of 20 to 22°C and humidity around 60%, and during this period, in terms of weight loss, side effects of the injection site and mortality were examined (22). In this study, the international standards of ethical rules for dealing with laboratory animals were observed.
The morphology of the vesicles was confirmed by electron microscopy and its size was determined between 40 - 200 nanometers (Figure 2)
The protein content of the extracted OMVs was estimated at 700 µg/ml. The profile of the proteins in extracted OMVs was observed by SDS-PAGE on polyacrylamide gel (Figure 3), presence of pertussis toxin, pertactin and filamentous hemagglutinin antigens in the extracted OMVs previously observed in the SDS-PAGE test confirmed by western blot test, using specific monoclonal antibodies, and completely specific bands were created for the antigens that were similar to the OMV isolated by the previous current method (Figure 4). OMV extraction was performed four times independently, and each time the results were similar in morphology and size and similar to those previously performed.
Figure 3. SDS-PAGE test of B. pertussis extracted with
a protein marker
With the injection of the extracted vesicles, the results of the pyrogeny test and the abnormal toxicity test were negative, so that no significant increase was observed in these tests, the increase in body temperature of any rabbit was not more than 0.7°C, which indicates that extracted OMVs was not pyrogenic. Also, in the test for abnormal toxicity, none of the animal models lost weight, no side effects were observed at the injection site, and all rabbits survived until the end of the seventh day.
Figure 4. Western blot test using PTX, FHA and PRN monoclonal antibodies. (1) OMV obtained from the modified extraction method, (2) OMV obtained from the current extraction method
The resurgence of pertussis occurs despite widespread vaccination, and given the importance of more prevention of the disease, there is much debate today about pertussis vaccines. Acellular pertussis vaccines (aP) was developed and replaced in many developed countries due to the side effects associated with whole-cell pertussis vaccines (wP). These aP vaccines contain inactivated toxin along with recombinant or pure B. pertussis bacterial antigens such as pertussis toxin, filamentous hemagglutinin, pertactin, and fimbriae. These vaccines also have disadvantages, including the effectiveness of these vaccines depends on the number and concentration of antigens present in its formulation. While wP creates immunity against a wide range of antigens. The aP does not inhibit colonization in the respiratory tract, and immunized individuals can be involved in the transmission of the disease in the population (23, 24).
Other limitations of the aP pertussis vaccines include a rapid reduction of antibodies in the body after vaccination and induction of humoral immunity by the vaccine. Because B. pertussis is an intracellular bacterium, the humoral response is not the acquired immune response required for immunity against pertussis and cannot produce long-term immunity, so the cellular immune response plays an important role in clearing B. pertussis. Therefore, there is a great need for a third-generation pertussis vaccine to cover the weaknesses of previous vaccines. A vaccine should not only stimulate Th2 responses, but also stimulates Th1 and Th17 responses, like wP vaccine (3, 25).
Outer membrane vesicles of bacteria play an important role in physiology, pathogenesis, and interaction between host and pathogenic agents, and contain surface immunogens in their structure (26, 27). On the other hand, since overtime after vaccination, genetic modification of pathogenic and immunogenic factors of the bacterium B. pertussis has caused genetic modification of strains to protect the bacterium from the host immune system (28).
It seems that the main stimulate pressure for vaccine development is due to the generation of vaccine-escape mutants, such as those that do not produce pertactin. Such strain became dominant in many countries (29-31). The ability of OMV for protection with the help of several components, including Ptx, FHA and PRN, it introduces OMV as a vaccine candidate that selective pressure does not affect the circulating strains, and reduce the possibility of strains escaping the vaccine, so OMV has acceptable properties that can be used as a good candidate for the pertussis vaccine (18).
In 2013, Fernandez evaluated the possibility of using OMVs extracted from B. pertussis as a vaccine candidate, and with the use of western blotting, the presence of pertussis toxin, pertactin, and fimbriae, were confirmed. LAL (Limulus Amebocyte Lysate) has been shown to have lower endotoxin levels than a vaccine licensed in wP (14).
The contents of the vesicles of the outer membrane of the Tohama strain were examined using various techniques in a study by Robert and colleagues. The results showed the presence of known surface immunogens, such as pertactin, adenylate cyclase, pertussis toxin, and lipooligosaccharide. Considering the inherent safety induced in the airflow pathway and the protective immunity in the mouse model, it was shown that the OMV obtained from B. pertussis is considered as a candidate for protection against pertussis (12).
In a study by Raeven in 2016, the possibility of using OMVs as a candidate for the pertussis vaccine was investigated. Comparing OMV-containing vaccines with wP and aP vaccines, high levels of antibodies were observed, and the results showed that the extensive and regulated humoral response makes OMV vaccine a potential and promising candidate (32).
In a study by Gillard in 2014, outer membrane vesicles obtained from the recombinant strain of B. pertussis were formulated with the Tdap vaccine as a vaccine candidate evaluated, and the results were compared with conventional commercial vaccines. The findings showed that Tdapomv induced Th1 and Th2 immune responses and provided protection against clinical isolates, while the common commercial Tdap vaccine-induced Th2 but weakened Th1 response and provided little protection against the pathogen. According to Tdapomv, it can be considered as a new vaccine (33).
Also, the Bexsero® (previously 4CMenB) vaccine, OMV isolated by the detergent in combination with the recombinant antigen, was introduced against Neisseria meningitidis serogroup B, developed by Bai et al. In 2011, and its immunogenicity was confirmed in clinical trials (34).
Extraction of the outer membrane vesicles of bacteria and their application in vaccine studies are developing nowadays. In previous studies, ultracentrifugation with a speed of 100000 g was used to isolate the outer membrane vesicles (12, 16, 33). Due to the unavailability of Ultracentrifuge in most research and academic centers in Iran, in this study, a new method for isolating OMVs of B. pertussis was presented. This method involves a series of simple high-efficiency steps that can be performed using high-speed centrifugation. Figure 2 confirms the natural spatial shape of the vesicles and the similarity of the extracted vesicles compared with previous studies. So that the size of the vesicles is 40-200 nm, which corresponds to the results obtained from the extraction of the vesicle using ultracentrifugation method (12, 16).
Also, the results of SDS-PAGE and Western using monoclonal antibodies show that the vesicles extracted in the new method are similar to the previous current method and contain important immunogens such as pertussis toxin, pertactin and filamentous hemagglutinin, which can induce the host immune system. The extraction was repeated four times, and the morphological similarity, size, and presence of antigens were observed in all cases. Also, the harmlessness of using the vesicles obtained by the pyrogeny test and the abnormal toxicity indicates that the sample is not contaminated with impurities during the extraction process. Therefore, it is possible to use it in clinical studies in animal models. This method has many positive advantages and is beneficial economically compared to the current method, hence it can pave the way for the extraction of vesicles on a large scale without the need for ultracentrifugation. OMVs obtained from the vaccinal strain of B. pertussis can be considered as a candidate for a new generation of pertussis vaccine alone or in combination with adjuvants for new acellular vaccines design in the future.
The authors thank all those who helped them writing this article.
Authors declared no conflict of interests.
Received: 2020/03/21 | Accepted: 2020/06/16 | ePublished: 2020/05/12
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |