BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1066-en.html
2- Department of Biology, College of Basic Science, Tehran Science and Research Branch, Islamic Azad University, Tehran, Iran
3- Department of Quality Control, Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Karaj, Iran
4- Department of Microbiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran ,
.
Staphylococcus aureus is one of the most important microorganisms that can colonize the human body and cause various types of human diseases by secreting virulence factors which are known as staphylococcal super antigens (SAgs) (1). SAgs are recognized by their ability to make a cross-link between some subsets of T Cell Receptor (TCR) and Major Histocompability molecule class II (MHCII) in a different position from MHC cleft (2, 3). It should be considered that different types of staphylococcal strains could produce different SAgs significantly including TSSToxin-1 (TSST-1), Staphylococcal Enterotoxin B (SEB) and Staphylococcal Enterotoxin C (SEC) (2). Among them, SEB is responsible for staphylococcal food poisoning in human and acts through stimulating the cytokine release and inflammation induction (4, 5).
Staphylococcal food poisoning is usually diagnosed by the clinical symptoms and also the probable presence of SEB in blood, urine, respiratory secretions, etc. The toxin can be detected by Enzyme-Linked Immunosorbent Assay (ELISA), chemiluminescence (CL), Reverse Passive Latex Agglutination test (RPLA-oxide), etc. Despite providing acceptable sensitivity, most of these common tests are time-consuming hence they need to improve in terms of increasing the sensitivity and decreasing the time consumption (6-9).
The Lateral Flow Assay (LFA) is one of the well-known commercial immunoassay methods providing a high sensitive and rapid approach to monitoring the infectious agents in blood, serum, urine, etc. Briefly, conventional LFA comprises capture antibodies embedded in test and control lines which are assembled on a nitrocellulose membrane and a detection antibody conjugated to colloidal gold which is loaded on a conjugation pad. In addition, gold nanoparticles have been used in various immunoassay techniques over the recent years because of their high stability, easy size controlling and high compatibility with biological molecules including antibodies. In this study, gold-conjugated antibodies are designed to develop a one-step immunochromatographic strip test in order to detect analyses.
Moreover, regarding the need for having high sensitive methods to precisely identify infectious compounds such as SEB, it would be indispensable to improve the sensitivity of LFA.
Recent studies have indicated that the sensitivity of conventional LFA can be enhanced approximately from 10 to 100-fold by adding a silver enhancement step to the test procedure hence, our laboratory has developed immunochromatographic rapid test strips similar to LFA to identify SEB. In the present study, the efficacy and sensitivity of conventional LFA is compared with the improved silver enhanced method of sandwich LFA. Thus, this new high sensitive method can be substituted for the previous SEB detection and measurement methods.
The Staphylococcal Enterotoxin A (SEA), SEB and SEC were purchased from SIGMA Company (S-4881). Colloidal gold was purchased from Arista Biological INC (CGUCG-0000). Two types of Anti-Entrotoxin B Staph (S222 and S624 Hytest) were used as conjugated and test antibodies, respectively. Goat-anti Mouse antibody was obtained from Hytest Company. The Silver Enhancer solution A and B (S5020 and S54145) used in the study were manufactured by Sigma Company.
Absorbent pad, Nitrocellulose membrane (AE98F) and Sample pad were purchased from Schleicher and Schuell Company. Backing card was purchase from G and L Company.
Colloidal Gold Conjugation of Detection Monoclonal Antibody
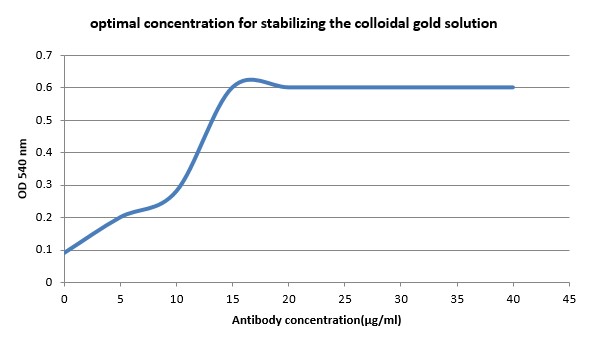
Based on the standard protocols (10), Anti-Entrotoxin B Staph mAb (S222) was diluted to Borax buffer to make 5, 10, 15, 20, 25, 30 and 40 µg/mL concentrations and the pH was adjusted to 9 with 0.2 M of k2CO3, then 1.0 mL of colloidal gold (pH=9) was added to 100 µL of each antibody dilution and incubated for 10 min at room temperature. Then, 100 µL of 10% NaCl was added to each vial and the color alterations were exposed. The lowest concentration with no color change was introduced as optimal concentration for stabilizing the gold solution (Figure 1). In order to stabilize conjugated antibodies, the solution was centrifuged at 8000×g at 4°C for 30 min. Conjugations were stored in Phosphate Buffer (PB) containing 50 mM phosphate (pH=9), 0.1 mm Tween® 20 and 1% Bovine Serum Albumin (BSA) at 4°C.
Production of Immunochromatographic Rapid Test Strip
Conjugated mAbs were diluted in 20 mM phosphate buffer and applied to the conjugation pad, in 2 μL/cm by biojet-quanti dispenser (BioDot, Irvine, CA.). Test line contained 0.5 μL/cm Anti-Enterotoxin B Staph and control line enclosed 0.5 μg/cm Goat-anti Mouse antibody. Various layers including sample pad, Conjugate pad, Nitrocellulose membrane, and absorbent pad were assembled on the backing card with a 2 mm overlap between them. Finally, as shown in Figure 2, all sheets were cut in 5 mm width using an automated cutter.
Conventional and Silver Enhancement Methods of LFA
In the conventional LFA as sandwich-type, SEB was detected by the quantity of the colloidal gold-conjugated mAbs accumulated on the capture antibody-assembled site resulting in appearance of two red lines on a single strip with a sensitivity about 10 ng/mL in purified toxin. After running the sample, sandwich-type strips were immersed in silver enhancer reagents A and B for 5 min. Finally, common washing processes in PB buffer and Tween® 20 solution was applied.
In order to detect SEB in the present study, we applied the capture mAb (S624) in the test line (0.5 µL/cm) and conjugated mAbs to the conjugate pad (2 μg/cm) while the control line only contained Goat-anti Mouse antibody (0.5 μL/cm). As shown in Figure 3, the complete white strip including the sample pad and the absorbent pad was able to detect SEB in the range of 120-10 ng/mL displaying two red lines in test and control margins. No reactivity was visualized with the buffer alone as a negative control.
Detection of SEB by Silver Enhanced-Method
Using silver, plays an important role in signal amplification. When the silver solution was added, the detection sensitivity in sandwich-type LFA increased approximately about 100-fold compared to the conventional LFA. As shown in Figure 4, the sensitivity of SEB protein detection was 10 ng/mL before the enhancement and increased to 0.1 ng/mL after silver enhancement.
Study of Immunochromatographic Strip Maintenance
In order to obtain the best consuming time after keeping the strips in the fridge, the quality of stored immunochromatographic strips was investigated. Our investigation suggested that the efficacy of the test strips for effective detection of SEB will remain unchanged for 24 months if they are kept in a dried place at 2-8°C. Moreover, to confirm the specificity of the test strips, other enterotoxins of S. aureus including SEA and SEC were tested by new enhanced LFA. Negative results were observed for both enterotoxins using present models of LFA (unpublished data).
SEB has been known as an important member of staphylococci enterotoxin group that causes clinical infection and food poisoning in human. To diagnose such a hazardous agent at the trace level, a number of high sensitive methods of detection have been widely developed (11, 12). Over the past decade, lateral flow assay has been considered as one of the best-known commercial alternatives to the immunoassay methods for detection of various analyses.
This method is based on the specific binding of antibody to antigen with an improved sensitivity and specificity as well as considerable reduction in the test time consumption (less than 5 minutes), sample preparation (~200 µL) and personnel training. These advantages make LFA ideal for initial screening tests but it should be mentioned that these tests are qualitative and other quantitative methods including ELISA and CLA should be used for detection of antigens. (10, 13-15).
The current LFA is based on micro fluidic mobility of molecules through the test strip and their accumulation on the test and control lines. Therefore, the time of passing the reagents and consequently, formation of red lines in the strips would be one of the important parameters likely affecting the sensitivity of our described method (16). In order to improve the performance of the test and shorten the time of immunoreactions, we stored the conjugated antibodies in PB containing 50 mM phosphate (pH=9), 0.1mm Tween® 20 and 1% BSA. This buffer contains BSA which offers specific features to the strips including prevention of nonspecific bindings of antibodies to the antigens as well as providing a higher viscosity to reagents leading to a longer SEB and gold-conjugated antibody interaction time (17).
Additionally, the proper selection of nitrocellulose paper sheet support layers with the smaller pore sizes led to decreased solution flow rate across the paper and subsequently increased interaction time of antigen-antibody. Thus, we chose a paper with the smallest pore size
(AE98F) and the slowest flow rate among the other different porous papers (AE98F, 99F and
100F). Moreover, using two monoclonal antibodies increased the sensitivity and specificity of antigen-antibody interaction in comparison with other immunoassay methods (18-21).
Finally, in the present study, silver enhancement was performed for signal amplification to maximize the sensitivity of immunochromatographic strips.
During silver enchantment, the colloidal gold which had been attached to the antibody, played the role of a nucleation site for the metallic silver deposition. The silvers layer increased the size of the gold and imparted a block color to the stained line in order to improve the optical extinction. As we found out, using the silver enhancement method, the sensitivity of the strip tests could be improved from 10 ng/mL to 0.1 ng/mL.
It should be noted that the minimal detection limits of the strip tests ranged between 1 to 5 ng/mL for antigen detection. In our strip tests, minimal detected limit was about 0.01 ng/mL. This sensitivity is considered advantageous for strip tests and a development in quick detection methods in this diagnostic range. It can be introduced as a suitable alternative for high sensitive Immunoassay tests. Based on technical information, minimal detection limits for most of the the ELISA kits ranges from 0.1 to 0.5 ng/mL. The mentioned limit can be lowered to 0.01 ng/mL only using some technical improvements such as Avidin-Biotin method. Therefore, sensitivity improvement by silver enhancement method (SEM) got these kits categorized in high sensitive immunoassays.
In the previous study, we produced colloidal gold based lateral flow immunoassay for SEB detection and compared its sensitivity and specificity with PCR method in S. aureus contaminated solutions (22). The kits used in the mentioned study were much less sensitive and had a minimum detection limit of approximately 10 ng/mL. Application of SEM significantly increased the sensitivity of the lateral flow immunoassay and lessened the toxin minimal detection concentration to 0.1 ng/mL. This method was used to increase the sensitivity of prostate specific antigen (PSA) detection kits by Rodríguez and the results suggested that it is very effective in increasing visual signals and subsequently effective in lowering detection limits (23). One of the important items in the test procedure is reaction time. By adding the resonator solution, the color of the lines become gradually bolder and bolder. Therefore, at a certain time, the reaction have to be stopped.
By increasing the reaction time, we had a dark background and test lines could not be distinguished from each other. Therefore, in cases where this method was used to detect toxins, the test procedure was stopped at a specific time and then results were documented. Since the test time was variable in different runs, it was very difficult to determine a reliable protocol for good precision and accuracy. To eliminate this problem, it is necessary to evaluate a negative test at the same time in all experiments in order to decide about the best reaction time.
In this study, we tried to simplify the test procedure by adding a silver enhancer pad on the test strip. The results indicated that the process was made much easier and the kit was more applicable. Therefore, a negative control line (containing neutral proteins such as IgG) was used in addition to the test line and the control line in this type of kits, so that the results of the test line and the control line could be compared simultaneously with the negative control for better decision about the test ending point.
Using rapid strip tests is broadly applicable for detecting various antigens and increasingly dispersed throughout the world. The increased sensitivity of these types of tests will allow them to be used in more important diagnostic cases. Using the silver enhancement method can increase rapid strip test sensitivity by resonating visual gold signals. In the present study, we used the colloidal gold-conjugated antibodies and the silver enhancement method for detecting SEB toxin increasing the test sensitivity relatively. Our results were indicative of usefulness of this method for rapid antigen detection present in nanogram scale.
The authors are grateful to Arvin Pazhoohan Noor Company (APN) for providing some of kit's consumables and Razi Vaccine and Serum Research Institute for providing the antigens used to evaluate Kit.
Authors declared no conflict of interests.
- Ramin Rashidi Nezhad, Seyed Mansour Meybodi , Razieh Rezaee ,Mehdi Goudarzi , Maryam Fazeli . Molecular Characterization and Resistance Profile of Methicillin Resistant Staphylococcus aureus Strains Isolated from Hospitalized Patients in Intensive Care Unit, Tehran-IranJanuary/February. Jundishapur J Microbiol. 2017 March; 10(3):e41666. [DOI:10.5812/jjm.41666]
- Xu SX, McCormick JK. Staphylococcal superantigens in colonization and disease. Front Cell Infect Microbiol. 2012;2:52. [DOI:10.3389/fcimb.2012.00052]
- Irina V. Pinchuk , Ellen J. Beswick and Victor E. Reyes. Staphylococcal Enterotoxins. Toxins. 2010; 2; 2177-2197. [DOI:10.3390/toxins2082177] [PMID] [PMCID]
- Ortega E, Abriouel H, Lucas R, Galvez A. Multiple roles of Staphylococcus aureus enterotoxins: pathogenicity, superantigenic activity, and correlation to antibiotic resistance. Toxins (Basel). 2010 Aug;2(8):2117-31. [DOI:10.3390/toxins2082117] [PMID] [PMCID]
- Asgarpoor D., Haghi F., Zeighami H.. Frequency of Enterotoxin Producing Staphylococcus aureus and Toxin Genes in Raw and Cooked Meat Samples. Infection Epidemiology and Microbiology. 2018;4(2):53-58. DOI: 10.29252/modares.iem.4.2.53
- Aguilar J.L, Varshney A.K, Wang X., Stanfor L., Scharff M, Friesa B.C. Detection and Measurement of Staphylococcal Enterotoxin-Like K (SEl-K) Secretion by Staphylococcus aureus Clinical Isolates. Journal of Clinical Microbiology.2014. 2536 -2543. [DOI:10.1128/JCM.00387-14] [PMID] [PMCID]
- Nagaragjapa S., Thakura M.S , Manonmani H.K. Detection Of Entrotpxigenic Staphylococci By Loop-Mediated Isothermal Amplification Method. Journal of Food Safety. February 2012.Volume 32, Issue 1, 59-65, [DOI:10.1111/j.1745-4565.2011.00344.x]
- Thad W. Vickery, Vijay R. Ramakrishnan, and Jeffrey D. Suh. The Role of Staphylococcus aureus in Patients with Chronic Sinusitis and Nasal Polyposis. Curr Allergy Asthma Rep. ; 2019(4): 21. doi:10.1007/s11882-019-0853-7. [DOI:10.1007/s11882-019-0853-7] [PMID] [PMCID]
- Wang D, Chen H., Li H., He Q., Ding X.H, Deng L. Detection of Staphylococcus aureusCarrying the Gene for Toxic Shock Syndrome Toxin 1 by Quantum-Dot-Probe Complexes. Journal of Fluorescence .July 2011, Volume 21, Issue 4, pp 1525-1530 [DOI:10.1007/s10895-011-0840-4] [PMID]
- Ching KH, Lin A, McGarvey JA, Stanker LH, Hnasko R. Rapid and selective detection of botulinum neurotoxin serotype-A and -B with a single immunochromatographic test strip. J Immunol -types. 2012 Jun 29;380(1-2):23-9. [DOI:10.1016/j.jim.2012.03.008] [PMID]
- Fujishima H, Okada N, Dogru M, Baba F, Tomita M, Abe J, et al. The role of Staphylococcal enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy. 2012 Jun;67(6):799-803. [DOI:10.1111/j.1398-9995.2012.02818.x] [PMID]
- Rubén Arturo Silvero-Isidre, Fátima Rodríguez-Acosta, César Rodrigo Cristaldo-Vargas, Genaro Américo Velázquez-Romero, José Félix Plans-Perrota, Rosa María Guillén-Fretes. Molecular Characterization of Staphylococcus aureus Isolates Obtained from Hemodialyzed Patients at the Hospital de Clínicas of Paraguay: A pilot study. IJMS.2017.vol.5, ssue 1 [DOI:10.5195/ijms.2017.172]
- Gucukoglu A, Onur Kevenk T, Uyanik T, Cadirci O, Terzi G, Alisarli M. Detection of Enterotoxigenic Staphylococcus aureus in Raw Milk and Dairy Products by Multiplex PCR. J Food Sci. 2012 Nov 5. [DOI:10.1111/j.1750-3841.2012.02954.x] [PMID]
- Cao H, Wang M, Zheng R, Li X, Wang F, Jiang Y, et al. (Investigation of enterotoxin gene in clinical isolates of Staphylococcus aureus). Nan Fang Yi Ke Da Xue Xue Bao. 2012 May;32(5):738-41.
- Singh A.K., Garber E.A, Principato M.C., Hall SH., Sharma S.K. Biotoxins and Food Safety. Biological Toxins and Bioterrorism .Toxinology , 2014, pp 185-210 [DOI:10.1007/978-94-007-5869-8_20]
- Kim JS, Taitt CR, Ligler FS, Anderson GP. Multiplexed magnetic microsphere immunoassays for detection of pathogens in foods. Sens Instrum Food Qual Saf. 2010 May 4;4(2):73-81. [DOI:10.1007/s11694-010-9097-x] [PMID] [PMCID]
- Han E., Ding L., Qian R., Bao L, Ju H. Sensitive Chemiluminescent Imaging for Chemoselective Analysis of Glycan Expression on Living Cells Using a Multifunctional Nanoprobe Analytical chemistry, 2012 . 84 (3), pp 1452-1458 [DOI:10.1021/ac203489e] [PMID]
- Lian W, Wu D, Lim DV, Jin S. Sensitive detection of multiplex toxins using antibody microarray. Anal Biochem. 2010 Jun 15;401(2):271-9. [DOI:10.1016/j.ab.2010.02.040] [PMID]
- Koch S, Wolf H, Danapel C, Feller KA. Optical flow-cell multichannel immunosensor for the detection of biological warfare agents. Biosens Bioelectron. 2000 Jan;14(10-11):779-84. [DOI:10.1016/S0956-5663(99)00051-2]
- Miroslav Pohanka. Current Trends in the Biosensors for Biological Warfare Agents Assay. Materials 2019, 12, 2303; doi:10.3390/ma12142303 [DOI:10.3390/ma12142303] [PMID] [PMCID]
- Yuanshun Zhao , Yonghong Zhang , Dongdong Lin , Kang Li , Chengzeng Yin, Xiuhong Liu , Boxun Jin , Libo Sun , Jinhua Liu , Aiying Zhang and Ning Li. Protein microarray with horseradish peroxidase chemiluminescence for quantification of serum a-fetoprotein. Journal of International Medical Research 2015, Vol. 43(5) 639-647 [DOI:10.1177/0300060515583075] [PMID]
- Gholamzad M, Khatami MR, Ghassemi S, Malekshahi ZV, Shooshtari MB. Detection of Staphylococcus enterotoxin B (SEB) using an immunochromatographic test strip. Jundishapur journal of microbiology. 2015 Sep;8(9). [DOI:10.5812/jjm.26793] [PMID] [PMCID]
- Rodríguez MO, Covián LB, García AC, Blanco-López MC. Silver and gold enhancement methods for lateral flow immunoassays. Talanta. 2016 Feb 1;148:272-8. [DOI:10.1016/j.talanta.2015.10.068] [PMID]
Received: 2020/02/23 | Accepted: 2020/07/19 | ePublished: 2020/07/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |