BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1050-en.html
2- Department of Cell and Molecular Biology & Microbiology, University of Isfahan, Isfahan, Iran ,
Melanin is a negative charge hydrophobic complex pigment that is a substance made of small particles virtually insoluble in the environment and is usually used for its color, protective or other characteristics. Pigments are of particular importance in many industries, including the food and pharmaceutical industries (1).
The importance of microbial pigments has been emphasized in a variety of applications including cosmetics, food, pharmaceuticals and textiles, and they also have cytotoxic, antioxidant, antimicrobial, anti-cancer, anti-tumor, and anti-combustion activities (3-5). It is also known as a potent antioxidant, antivirus, and antibiotic, in addition to being able to protect organisms against toxic free radicals, protect against pathogenic bacteria, and regulate heat. Melanin can be produced in bacteria by chemical synthesis methods based on tyrosine oxidation and enzyme catalysis. One of the problems with the use of melanin extracted by microorganisms is the presence of impurities of toxic secondary metabolites that cannot be used in medicine and food. Other problems include insolubility in water and solubility in organic materials. Melanin is classified into three forms: eumelanin, pheomelanin, and neuromelanin (7, 4).
Melanin producing bacteria include some species of Aeromonas, Streptomyces, Bacillus, Vibrio, and Alteromonas (3, 11-13). Melanin produced from the bacteria Azotobacter chroococcum and Burkholderia cenocepacia has strong antioxidant properties and therefore they can protect themselves against environmental free radicals (14).
Water-soluble brown pigment pheomelanin can be synthesized by Pseudomonas species including Pseudomonas aeruginosa, Pseudomonas alcaligenes, and Pseudomonas putida using a tyrosinase mechanism within 24 to 48 hours (5, 16, 17).
In this study, the bacterium Pseudomonas stutzeri UIS2 was isolated to produce melanin in the presence of L-tyrosine. This is the first report of a P. stutzeri bacterium that produces high levels of melanin only in the presence of L-tyrosine. Melanin pigment extraction and its structure have been studied using spectroscopic analysis methods and its protective properties against sunlight and its antioxidant inhibition have been determined.
To separate the melanin producing bacteria from the soil sample, 1 g of each sample was added to 10 mL of 8.5% normal saline buffer in a 50 mL flask, and was shaken (60 rpm) for 30 min at 37°C (18). Initial identification was done by gram staining, morphological and biochemical tests (21–19). Melanin production during bacterial growth was monitored by spectrophotometer at 400 and 600 nm in comparison with melanin standard (7). KOH test was used to confirm the staining. Biochemical tests were used to identify the isolates initially.
After extraction of the isolated strain gene by boiling cell mass grown in Loria-Brittany broth culture, molecular identification using primers 1492R 5’-CGGTTACCTTGTTACGACTT-3’ and 27F-YM 3′-AGAGTTGCT-3AG-AGAGTTGCT-5’.
16SrRNA gene fragment amplification with about 1500 open pairs was performed by PCR and amplified fragment sequencing. Sequence of the above product was blasted in NCBI and MEGA-6 software was used to draw the phylogenetic tree. To do this, the homologous strain sequences were first extracted from the NCBI site and then sequenced by the Muscle program in MEGA-6 software (7).
The melanin pigment was then extracted and then purified. To determine the potency of a sunscreen with a sun protection factor (SPF). To determine the extracted SPF of melanin as a sunscreen metabolite, it was first dissolved in a specific concentration in ethanol and then its UV absorption from a wavelength of 290 to 320 nm at a distance of 5 nm was measured by spectrophotometer and calculated by the following equation (24):
The free radical scavenging activity of the pigment extract of Melanin bacterium was measured by 2, 2-diphenyl-1-picrylhydrazyl (DPPH) using modified Brand-Williams et al. method. Ascorbic acid was used as standard composition and tested in three replications. The percent inhibition of DPPH compound was calculated using the following equation (4):
DPPH inhibition (%) = (control absorbance _ test absorbance) × 100
Among the grown bacteria, gram-negative bacillus, strain 2 UIS, was isolated from soil samples of Isfahan University Park with the ability to produce melanin on the L-tyrosine-containing medium. The formation of a brown or black area around the isolated colonies in the culture meThe biochemical properties of this bacterium were able to grow at acidity of 8.5 and at optimum temperature of 35°C under aerobic condition, catalase and oxidase positive conditions, indole, MR, VP and negative H2S, negative gelatinase, positive citrate intake, casein hydrolysis and positive. The bacterial colonies were pink on MacConkey agar, yellow on nutrient agar, and brown on tyrosine agar.
The gel image corresponding to the PCR strain isolated in Figure 2 shows the product of 1500 matched pairs against the DNA marker. The sequences obtained from the PCR product after blast in NCBI showed 99.92% similarity with P. stutzeri (accession number AB680324.1). The isolated strain of melanin pigment generator was accessed at NCBI GenBank National Biotechnology Information Center under accession number MG519615. The phylogenetic tree was constructed by multiple sequences with evolutionary intervals by software. Tree topology was analyzed by bootstrap analysis of 100 datasets using MEGA6.1 software (Figure 3). Its phylogenetic tree also shows the close phylogenetic relationship of the sequenced strain with P. stutzeri.
Melanin pigment was produced in the resting phase of bacterial growth after 70 h with the appearance of black pigment in broth medium. Generally, melanin produced by this bacterium was about 600 mg/L. Melanin purity was observed by observing the absorbance peak at 210 nm (Figure 4).
DPPH inhibition was obtained by measuring absorbance at 516 nm at about 75% and close to standard ascorbic acid (78%) as shown in Figure 5.
The results of UV absorption at different wavelengths and SPF calculation are presented in Table 1. The SPF of melanin obtained from P. stutzeri UIS2 strain was found to be 49.05 as the inhibitor of ultraviolet radiation.
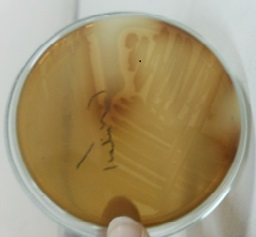
Figure 1. P. stutzeri bacterium after 24 h in a 2 g / L l-tyrosine agar medium incubated at 30°C in degrading l-tyrosine and primary melanin production.

Figure 2. PCR image of 16S rRNA gene of some isolated melanin producing strains: (1) UIS19 strain, (2) UIS2 strain, and (3) 1kb marker DNA.
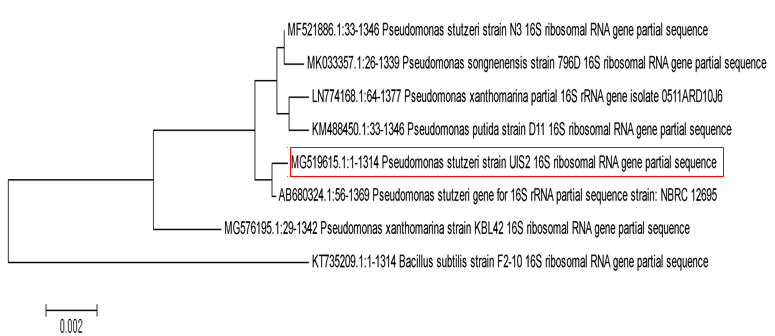
Figure 3. Phylogenetic tree of the melanin-producing UIS2 strain isolated (MG519615 Pseudomonas accession number isolate in the box marked whose 16S rRNA sequence was 99.92% similar to that of P. stutzeri bacterium accession number AB680324.1).
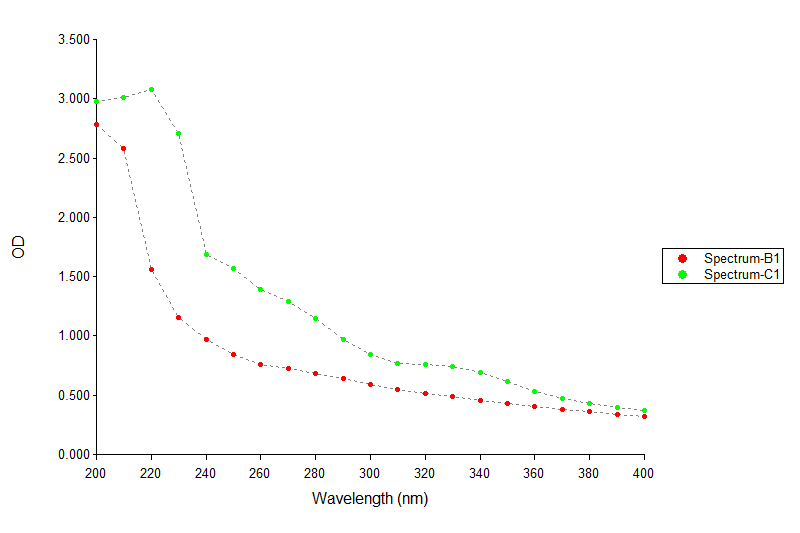
Figure 4. Diagram of absorbance length of standard melanin (B1) and pure melanin UIS2 (C1) strain with UV spectrophotometer
Table 1. Optical Adsorption Results of Melanin Isolated P. stutzeri UIS2 Strain by Melanin Dissolution in Methanol for Determination of SPF
| λ(nm) | Abs | EE.I | EE.I × Abs |
| 290 | 0.686 | 0.015 | 0.01029 |
| 295 | 0.672 | 0.0817 | 0.0549024 |
| 300 | 0.648 | 0.2874 | 0.1862352 |
| 305 | 0.831 | 0.3278 | 0.2724018 |
| 310 | 0.794 | 0.1864 | 0.1480016 |
| 315 | 0.642 | 0.0839 | 0.0538638 |
| 320 | 0.632 | 0.018 | 0.011376 |
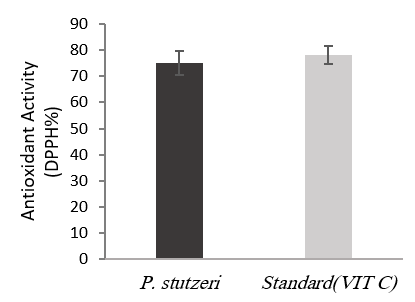
Figure 5. Antioxidant activity of melanin produced from P. stutzeri UIS2 in comparison with standard ascorbic acid at concentration of 20 mg/L in DPPH test
Pseudomonas stutzeri is a gram-negative bacillus with flagella and grows under aerobic conditions on medium containing starch and maltose and is unable to degrade arginine and glycogen. Its difference with other Pseudomonas strains is that they do not produce fluorescence pigment and are very similar to those of Pseudomonas alcaligenes, and Pseudomonas putida. This bacterium is difficult to isolate because of limited nutrient requirements for growth and is well grown in medium containing low ammonium nitrate and incubation temperature of 37°C.
The new colonial form of this bacterium differs from that of other Pseudomonas spp. The colonies are dry, hard, wrinkled and branched, but it is easy to remove from the surface of the solid medium and changes in shape and color after some time. For this bacterium, a very wide growth temperature range between 4 and 45 °C has been reported (26).
Melanization has important roles such as protecting and adapting to various physiological and chemical stressors such as temperature, radiation, humidity, and toxicity by various contaminants in microorganisms (33).
Maximum melanin production in isolated UIS2 strain Pseudomonas was about 600 mg/L, which is 3.6 times higher than that of Streptomyces bikiniensis with 166 mg/L melanin in culture medium containing yeast and peptone extracts. In Yarrowia lipolytica yeast the production of melanin is about 160 mg/L, and Klebsiella GSK mediated by tyrosine is reported at about 130 mg/L (34). On the other hand, HMGM-7 strain of Pseudomonas stutzeri produces about 6.7 g/L of melanin under optimum conditions (20), which is higher than the native isolate in this study due to the optimization performed.
The sun protection factor of the strain isolated in this study was 40.05 which is very good compared to the synthetic and natural ingredients used in cosmetics. It is also worth noting that the production of melanin as a solar UV blocker using non-pathogenic environmental bacteria in the presence of L-tyrosine is much faster and with higher production rates than metabolites produced by algae and cyanobacteria.
Pseudomonas stutzeri was isolated as a melanin producing bacterium in this study, which was able to grow in a simple medium (nutrient agar) containing l-tyrosine and melanin synthesis. The biological properties of the isolated melanin strain have been determined for use in industry. Melanin pigment of this strain showed high antioxidant activity against ultraviolet radiation and oxidative stress ROS. Isolated melanin can be used in cosmetics, pharmaceuticals, agriculture, and environmental contaminants. Its antioxidant properties can inhibit DNA damage and other biological compounds. In addition, melanin is used as a skin protection agent in creams and cosmetics.
The authors would like to thank the research assistant of the University of Isfahan for the financial support of this research which was a part a doctoral thesis.
Authors declared no conflict of interests.
Received: 2020/01/28 | Accepted: 2020/02/25 | ePublished: 2020/03/23
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








