BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1014-en.html
2- Department of Microbiology, Ahar Branch, Islamic Azad University, Ahar, Iran ,
.
The most common malignancy of cancer in women is cervical cancer, which is the third most common cancer of the genital tract after breast cancer (1). HeLa is a type of immortalized cell line used in scientific research as the oldest and most common category (3). This cancer is caused by the irregular growth of the cervical epithelial cells and the continuous loss of cells worldwide. The most important factor is Human papillomavirus (HPV). For cancer research studies, HeLa cancer cells are used, the advantages of which include the ability to proliferate indefinitely and endure long passages (5). Various studies have shown that probiotics have an effective role in the fight against cancer. Their anticancer mechanisms include suppressing mutations, inhibiting cancer-causing compounds and tumors, and strengthening the immune system. In 6% of cases, HPV infection is the major factor that can be prevented using probiotics (6). The anticancer effects of probiotics are by preventing the conversion of procarcinogen to carcinogen, binding and inactivating mitogenic compounds, reducing the growth of pro-carcinogenic bacteria, reducing mitogen uptake and enhancing immune function (7). One of the functional mechanisms of probiotics, including Lactobacillus, is the induction of anti-cancer apoptosis (8). Apoptosis, which occurs in response to various stressors such as physiological, pathological or cytological stimuli in the body (9), plays an effective role in controlling the physiology of the body and many pathological conditions. Resistance to apoptosis is one of the hallmarks of cancer and reduced susceptibility to apoptosis results in increased threshold therapy for classic cases such as chemotherapy and radiotherapy (10).
Most anticancer drugs induce apoptosis through the mitochondrial pathway (12). Caspases are proteases that act as essential initiators and performers of the apoptotic process. Classically, the caspase 3 cascade is most likely initiated by autoproteolysis by breaking the so-called initiator caspases (2, 8, 9, 10, 12). Initial caspases break down and activate caspases (caspases 3, 6, and 7). This results in an apoptotic process (13).
Importance of Caspase-9 is that it is upstream signaling of Caspase 3 and is stimulated by 1-Apaf / cytochrome c and is inhibited by the oncogene product Akt (16). Two major pathways for apoptosis have been identified:
1: responds to foreign pathway (death receptor dependent pathway) and internal factors such as DNA damage;
2: Internal pathway (mitochondrial pathway). The external pathway of apoptosis begins through death receptors and leads to activation of caspase 8. Activated caspase 8 can directly activate executable caspases, and activated caspases such as caspase 3 are subsequently activated by the initiator caspases and trigger the caspase cascade. (17). The aim of this study was to investigate the effect of Lactobacillus brevis on apoptosis and casp8 (casp8, casp3) gene expression in HeLa cancer cells.
In this cross-sectional study, sampling of vagina was performed on women referring to Alzahra Maternity Hospital (Tehran, Iran) from ….. to ….. Eight out of 70 samples were positive for L. brevis bacteria which were isolated after MRS agar culture. To maintain the samples at -70°C, a new 12-hour culture of L. brevis was prepared in MRS agar culture medium. Then a standard bacterial loop was removed and 25% glycerol was added to MRS broth. After 12 h incubation, the samples were gradually cooled and placed in -70°C freezer.
Bacterial identification in the collected samples began with DNA extraction. DNA was extracted by agarose gel electrophoresis and spectrophotometric methods. PCR was performed on DNA. The primers sequence was designed by oligo5 software for PCR (Table 1).
In this project, HeLa cancer cells were obtained from Pasteur Institute of Iran's Cell Bank. Cells were cultured in flasks containing 5 mL RPMI1640 medium supplemented with 10% Fetal Bovine Serum (FBS), antibiotic penicillin 0.1 µg / µL and streptomycin 0.1 µg / µL.
To determine the viability test, cells were stained with trypan blue. Cell counting was performed. The mean was multiplied by 104 and dilution coefficient and the number of cells was obtained in one milliliter of solution.
Microculture Tetrazolium Test (MTT) and Dapi staining was done. Then the RNA was extracted. Samples were randomly electrophoresed on agarose gel to evaluate the quality of the extracted RNAs. For this purpose, 2% gel was made and then the samples were electrophoresed at 80 V for 1 hour.
RNA was extracted from cDNA to measure gene expression changes by real-time PCR. The primers used were designed by oligo 5 software and then blasted by the NCBI (http://www. ncbi. nlm. nih. gov).
The collected data were analyzed using SPSS 19 (SPSS Inc., Chicago, IL., USA).
The aim of this study was to investigate the mechanism of L. brevis as probiotic inhibitory effect on HeLa cervical cancer cells and its effect on expression of casp8, casp3 and ak1 genes. The results of the relevant tests were as follows:
Toxicity of Isolated Bacteria on HeLa Cancer Cell
In order to evaluate the toxicity of Lactobacilli bacteria on HeLa cells, the number of cells needed for the experiment were optimized first, and then cultured in 10,000 wells per 96 well plate. When the number of cells was doubled, the bacteria were cultured with increasing concentrations and then treated for 24, 48 and 72 hours. After 4 h no effect was observed but in the 12 h of treatment, lactobacillus bacteria were observed. During 48 hours of incubation, results were greater than IC50. The results of MTT are shown in Figure 1.
Table 1. Sequences of primers used to identify Lactobacillus brevis bacteria
| Gene | (5’-3’) | Product size |
| 16s rRNA |
F: GAACGCGAAGAACCTTAC
|
1500 bp |
| R: GCGTGTGTACAAGACCC |
Table 2. Characteristics of primers used
| Primer’s name | Primer Sequence | |
| CASP3 | Forward | 5-TACCCTGAAATG GGCTTGTGT -3’ |
| Reverse | 5-ﹶ GTTAACACGAGTGAGGATGTG - 3’ | |
| AKT1 | Forward | 5’-TGCCCACACGCTTACTGAGA-3’ |
| Reverse | 5′‐CAAGTAGTCCAGGGCGGACA‐3′ | |
| Casp8 | Forward | 5’- - CCCAAGAGGAACAGCGATAAG -3’ |
| Reverse | 5’- GGTCGATGGTGGTGTCAAAG -3’ | |
| GAPDH | Forward | 5 -CGGTGGATCCCCTTTATTG-3 |
| Revers | 5-CTAACCAGGAATTCCGATG-3 |
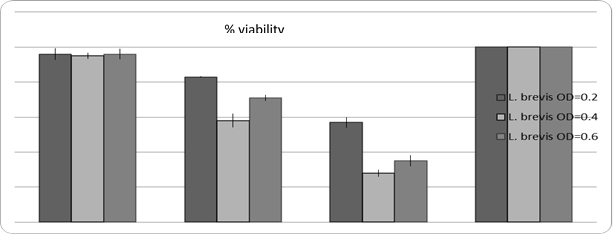
Figure 1. The effect of different time treatments on Lactobacillus brevis Culture.
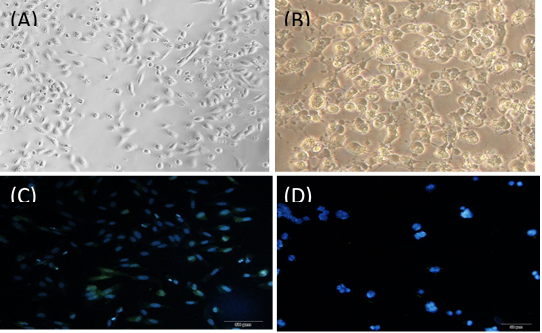
Figure 2. Staining of cells treated with DAPI staining
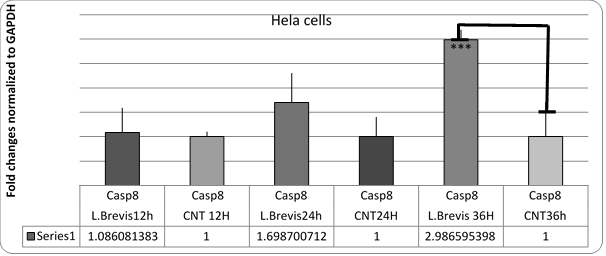
Figure 3. Diagram of changes in Casp8 gene expression in HeLa cells affected by L. brevis.
***; (P = 0.42)
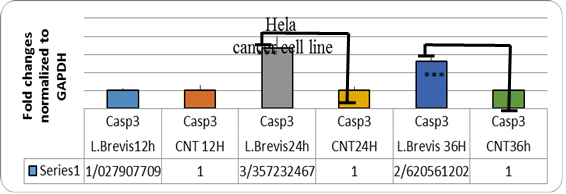
Figure 4. Graph of expression of CASP3 gene in HELA cells affected by L. brevis.
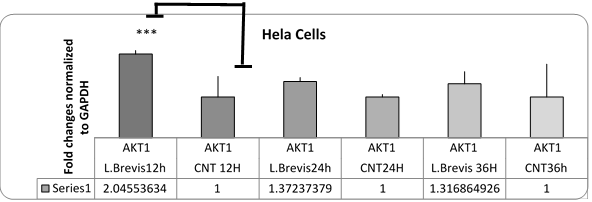
Figure 5. Chart of changes in akt1 gene expression in HeLa cells affected by L. brevis (P = 0.42). The akt1 gene had increased expression in the early hours due to the resistance of the cancer to apoptosis.
DAPI staining
The results of DAPI staining to evaluate apoptosis from different treatments are as follows (Figure 2). Treated cells compared to non-treated cells (with killed bacteria) entered apoptosis and smaller nuclei have formed.
To evaluate gene expression changes, HeLa cells were affected by IC50 concentration of lactobacillus bacteria obtained in MTT assay. RNA was then extracted from these cells.
HELA cells were treated with IC50 concentrations of lactobacillus isolates for 12, 24 and 36 hours, respectively. P-value<0.05 was considered significant.
In addition, in order to ensure the apoptosis of the cells upstream of the caspase 3 gene, caspase 3 was also investigated. There was a significant increase in this gene. The akt1 gene had increased expression in the early hours due to the resistance of the cancer to apoptosis.
Since akt1 gene plays an important role in the biology of cervical cancer, it has also been evaluated with regard to its anti-apoptotic effect to make it more reliable. As shown in the Figure 5, the akt gene did not increase significantly at 24 and 36 h, and the cells would be allowed to initiate apoptosis.
he main feature of cancer cells is uncontrolled cell proliferation. It also provides resistance to programmed death, so a factor that induces apoptosis in cancer cells can be recognized as an anticancer drug (20). There are also foods that can have a positive impact on the control of cancer cell growth. Therefore, dietary compounds and their role in human health have attracted the attention of many scientists.
Probiotics are some of the non-pathogenic organisms in the food that are present in the digestive system. These microorganisms also have beneficial effects on the health of the host. Certain probiotics have been reported to have anticancer activities (21). The results of tests on HeLa cell line and examination of genes involved in apoptosis of these cells showed that Lactobacillus brevis has the ability to inhibit the growth of HeLa cancer cells. Extract of this bacterium increased casp8 and casp8 gene expression at 24h and 36h (P> 0.05). However, no significant increase in akt1 gene expression was observed. Since caspase gene is a pro-apoptotic protein, apoptosis of cells was predicted by treatment with bacterial extract. Also, apoptosis of cancer cells by treatment of these bacteria with DAPI staining showed the antibiotic effect of the isolated probiotic.
Various studies on probiotics have shown that probiotics are living microorganisms that have a positive effect on improving the pathogenicity of the host and balance the number of microorganisms in the gut and strengthen the immune system. Probiotics play an important role in improving health. In the absence of these bacteria, the balance of the intestinal flora becomes disrupted and disease and inflammation occur (22).
Research has also shown that vaginal lactobacilli are not only effective in preventing bacterial and fungal infections, but also in controlling and preventing viral and cancer infections. According to these results, enhancing and preserving the microbial flora of vaginal Lactobacillus is one of the most important and low cost methods of cancer prevention. Lactobacillus family bacteria are commonly found as probiotics in yogurt and other dairy products. The regulating and stimulating properties of these bacteria on the host immune system have been well documented in other studies (23).
The results of tests performed on HeLa cell line and cell treatments and the study of genes involved in apoptosis in this study showed that the expression of caspase gene that was changed after treatment with Lactobacillus extract Further studies have been done on this probiotic bacterium and its anticancer properties that have proven the nutritional value of this probiotic. Cancer cells divide through a series of regulated processes, and the remainder of their cells are absorbed by adjacent tissues and immune cells. Numerous studies have shown that probiotic bacteria can play an important role in regulating apoptosis through internal and external pathways that have potential mechanisms for cancer prevention. This is consistent with the present study (24).
Investigating the influence of Lactobacillus brevis on gastric gastritis healing caused by Helicobacter pylori mouse model Orcid et al., (2018) showed that inflammation and remission were reduced in groups infected with L. brevis infection. The eradication rate of H. pylori infection in the treatment group showed a decrease in inflammation after histological examination. The results of the Orcid et al. study are consistent with our study (25).
Recent studies have confirmed the presence of caspases and AKT signaling pathway in cervical cancer cell and cancer cell death. As a result of this study, Akt1, a major mediator of the signaling pathway, which induces cancer cell survival, inhibits apoptosis of cancer cells, and drugs and therapies that inhibit AKT signaling pathway induce apoptosis of cancer cells. This is also consistent with our study (27). Studies have shown that AKT is activated by a number of growth factors through the RAS, PI3 signaling pathway. AKT has been reported as a result of activation of cervical cancer. In high grade cervical cancers, gene is highly active. In another study, of the 44 cervical cancer samples, 33 reported active AKT. This finding suggests that activation of AKT is a common process in cervical cancer. The AKT pathway is an important target for the study of cervical cancer. The findings of this study are consistent with our study (28).
The issue that active form of caspase-9, cleaves procaspase 3 and induces caspase 3 activation may be important in apoptosis. In line with the importance of these two caspases in apoptosis, Sanguinarine increases caspase 3 and 9 treatment about 6 to 7-fold. In fact, these caspases are activated in human colon HT-29 cells, indicating that Sanguinarine induces apoptosis by a caspase-dependent pathway in this cell line (29). In a study in 2015, Jafari et al. it was reported that caspase 3 is a key regulator of apoptosis and is associated with the incidence of apoptosis in breast cancer. Therefore, it can be considered as an important marker for predicting response or resistance to therapeutic drugs (chemotherapy). The results of their study are consistent with our study (30). Concerning gastric cancer, it has also been reported that caspase 3 expression levels in gastric tissue of gastric cancer patients are lower than in normal gastric mucosa (31).
Lactobacillus brevis can be biologically safe for the development of a novel, high-impact, low side-effect therapeutic strategy. On the other hand, side-by-side treatment and prevention against cancer will be cost-effective.
I would like to sincerely thank the Vice-Chancellor for Education and Research of the Islamic Azad University of Ahar Branch. I would like to thank all those who helped me during this research. It is worth noting that this article is the result of a thesis by Ms. Neda Chobdar at Islamic Azad University, Ahar branch, Iran.
Authors declared no conflict of interests.
Received: 2019/12/16 | Accepted: 2020/02/14 | ePublished: 2020/03/24
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








