BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-977-en.html
2- Department of Microbiology, Stamford University Bangladesh.51 Siddeshwari Road, Dhaka-1217. Bangladesh ,
.
Human body harbors different types of microorganisms located at different sites. In some areas there prevails a complex mixture of microorganisms which have inter-connections and function together. The functional characteristics of the complex of microbes often differ from their individual characteristics. In human body one such area harboring a complex mixture of microorganisms is oral cavity. Nearly 700 types of microorganisms have been found to be present in the oral microbe complex ecosystem which mainly takes part in dental diseases mostly like dental caries, periodontitis etc. which are very common infections in human seeking dental treatments (1-6). Some microorganisms of oral cavity can also cause systemic diseases like pneumonia, endocarditis, osteomyelitis etc. if they disseminate via the blood stream to distal body parts (7,8). Dental diseases like tooth decay has been prevailing from prehistoric ages which drastically increased after the increased consumption of refined sugar (9). W. D. Miller was the first pioneer who proposed the presence of oral organisms or germs in oral cavity of human who can ferment carbohydrates into acid which is responsible for tooth decay. Streptococcus mutans was the first bacteria which was isolated by J. K. Clarke (10,11). In different studies it was found that Actinomyces species can cause dental diseases (12). Dental plaque occurs due to the rapid proliferation of dental microorganisms producing biofilms which was first seen by Sir Antony Van Leuwenhoek under the microscope (13). It has been revealed that the microbes in dental plaque are responsible for the initiation of dental decays by producing acidic byproducts. Some common species found to be present in dental plaque includes Streptococcus spp., Actinobacillus spp., Actinomyces spp., Porphyromonas spp., Treponema spp. etc. (14). Dental plaque is formed by the actions of biofilms which is a mass of bacterial community where different microbiota can inhabit together in a gelatinous matrix. Because of the gelatinous material, the microbes are resistant to external hazards. There is also extensive metabolites exchanges, complex interactions among themselves which is unique for biofilms and cannot be seen when these species are living alone (15). Supragingival plaque has been showed to be dominated mostly by Gram-positive bacteria like Streptococcus sanguinis, Streptococcus mutans, Streptococcus salivarius, Streptococcus mitis and lactobacilli. Subgingival plaque is harbored by gram-negative anaerobic bacteria like Aggregatibacter (Actinobacillus) actinomycet-emcomitans, Tannerella forsythia, Fusobacterium nucleatum, Eikenella corrodens, Campylobacter spp., Capnocytophoga spp., Porphyromonas gingivalis, Prevotella intermedia, and oral spirochetes like Treponema denticola (16). The treatments for dental diseases generally include surgical approaches like root canal, filling, surgical removal of teeth and so on. But at the same time it is important to detect the cariogenic bacteria which damage and dimineralize the tooth by producing acids and enhancing the ways of re-mineralization of tooth. A new approach to decrease the dental problem is to introduce probiotics in mouth instead to killing all microbes from oral cavity. Because oral cavity includes a wide variety of microbial population, killing some microbes may not lessen the problem, because other oral microbial flora can replace in the new vacant places. But if we use probiotics which don’t initiate plaque formation and also can produce bacteriocin type products which can kill other plaque forming bacteria can be more useful in maintaining healthy oral cavity (17,18). In our current study we randomly selected 9 patients who came to seek dental assistance. The symptoms were first enlisted and microbiological analysis was performed to determine the presence of microorganisms in the specific infected area of oral cavity and then necessary treatment was suggested.
Sample collection and processing
Firstly, the physiological conditions (symptoms associated with dental diseases) for which patients were seeking dental check up along with the specific clinical symptoms identified by the healthcare personnel were enlisted. For microbiological analysis, dental plaque samples from twelve patients were collected in September, 2018 after asking their consent in a local dental health care center in Mirpur, Dhaka, Bangladesh. Among them one sample was from healthy individual as control. There was no ethical code for the research and no further involvement of any organization regarding the ethical code was not necessary. Sampling was done by using sterile forceps or toothpicks and transferred into 2 mL of reduced transport fluid medium (0.4% agar, 0.15% thioglycollate / phosphate buffered saline and stored at 4oC until plating onto specific agar plates. While studying the sample it was transferred to 9 mL thioglycollate broth and incubated for 24 hours and then serially diluted for inoculation into specific plates for identification of several dental plaque forming bacterial isolates in case of spreading plate technique. And for streaking, the original sample that was collected was used to directly inoculate onto the agar plates (19,20).
Isolation of Streptococcus spp.
For isolation of Streptococcus spp., Mitis Salivarius Agar (MSA) was used. This media is especially used for isolating Streptococcus mitis, Streptococcus salivarius from mixed cultures found in dental plaque samples. After incubation period at 37oC for 18-48 hours, presence of specific microorganisms was observed. Streptococcus mutans produce undule-shaped colonies, with frosted-glass appearance due to producing dextran from sugar. Streptococcus mitis could be identified as light blue colored, small and flat colonies. And Streptococcus salivarius could be identified as sticky, mucoid, gum-drop like colonies.
Isolation of Lactobacillus species
BD LBS Agar (Lactobacillus identification agar) is used for the detection of Lactobacillus spp. This media is also known as Rogosa Agar. Collected dental samples were introduced directly onto this agar followed by four quadrant streaking method to determine the presence of Lactobacillus spp. The dental samples were collected by dental floss can also be inoculated by spreading 0.1 mL over the media. Inoculated plates were incubated at 37oC for 48 to 72 hours in anaerobic condition. After incubation, Lactobacillus spp. could be determined by observing medium to large sized, white colonies onto the media.
Isolation of Actinomycetes
Collected dental floss sample (0.1 mL) was inoculated onto Actinomycetes Isolation Agar and incubated at 37oC for 3 to 7 days because they are slow growing microbes. After incubation Actinomycetes spp. can be identifies as mucoid, fungi like filamentous growth characteristics onto the agar plates.
Isolation of Spirochetes
For isolating Treponema species like Treponema amylovorum, Treponema denticola, Treponema maltophilum, Treponema medium, Treponema pectinovorum, Treponema socranskii, and Treponema vincentii etc. which can be found in dental plaques are cultured in NOS Spirochete medium. After inoculation onto the NOS medium by streaking, the plates were incubated at 37oC anaerobically for 7 days. In current study we inoculated dental plaque sample onto the NOS agar medium and the Spirochete grew in NOS agar as a white, hazy, cottony growth.
Isolation of Porphyromonas gingivalis
P. GING media has been used to isolate Porphyromona gingivalis from dental plaque samples. The media was inoculated directly with the broth where the dental plaque sample was directly suspended and then streak plate method was applied. The plates were then placed in an anaerobic atmosphere and incubated at 37oC for 48 hours.
Isolation of Capnocytophaga species
VCAT media was used in this study to isolate Capnocytophaga spp. from dental plaques. Samples were streaked onto VCAT agar and incubated anaerobically at 37oC for 48 hours. Colonies are convex or flat and often slightly yellow, show regular or spreading edges.
Table 1- Symptoms and microorganisms responsible for dental plaque formation among patients.
| Patient No. | Clinical Symptoms | Actinomycetes | Lactobacillus spp. | Capnocytophaga spp. | Streptococcus mutans | Treponema denticola | Porphyromonus gingivalis | Treatment suggested |
|---|---|---|---|---|---|---|---|---|
| 01 |
|
- | ++ | - | ++ | - | - | Left 6 Root cannel treatment performed followed by porcelain crown. Scaling and oral hygiene instruction done. Suggested for checkup after 3 months. |
| 02 |
|
- | ++ | - | ++ | - | - | Left 6 Root cannel treatment performed followed by porcelain crown. |
| 03 |
|
- | ++ | - | ++ | - | - | Left 4,5 Root cannel treatment performed followed by porcelain crown. Scaling and oral hygiene instruction done. Suggested for checkup after 3 months. |
| 04 |
|
- | ++ | - | ++ | - | - | Right Upper 7 Root cannel treatment performed followed by porcelain crown. Scaling and oral hygiene instruction done. Suggested for checkup after 3 months. |
| 05 |
Peripheral X-ray showed horizontal and alveolar bone loss.
|
- | - | - | - | ++ | ++ | Scaling done. Systemic abnormalities should be treated and oral hygiene instruction done. Suggested for checkup after 3 months. |
| 06 |
Peripheral X-ray showed horizontal and alveolar bone loss.
|
- | - | - | - | ++ | ++ | Scaling done. Systemic abnormalities should be treated and oral hygiene instruction done. Suggested for checkup after 3 months. |
| 07 |
|
++ | - | ++ | - | - | - | Scaling, Oral hygiene instruction, Review after 6 months. |
| 08 |
|
++ | - | ++ | - | - | - | Scaling, Oral hygiene instruction, Review after 6 months. |
| 09 |
|
- | - | - | - | ++ | ++ | Scaling done. Systemic abnormalities should be treated and oral hygiene instruction done. Suggested for check up after 3 months. |
| 10 |
|
- | - | - | ++ | - | - | Left 7 Root cannel treatment performed followed by porcelain crown. |
| 11 |
|
++ | - | ++ | - | - | - | Scaling, Oral hygiene instruction, Review after 6 months. |
| 12 |
|
- | - | - | - | - | - | No treatments needed. |
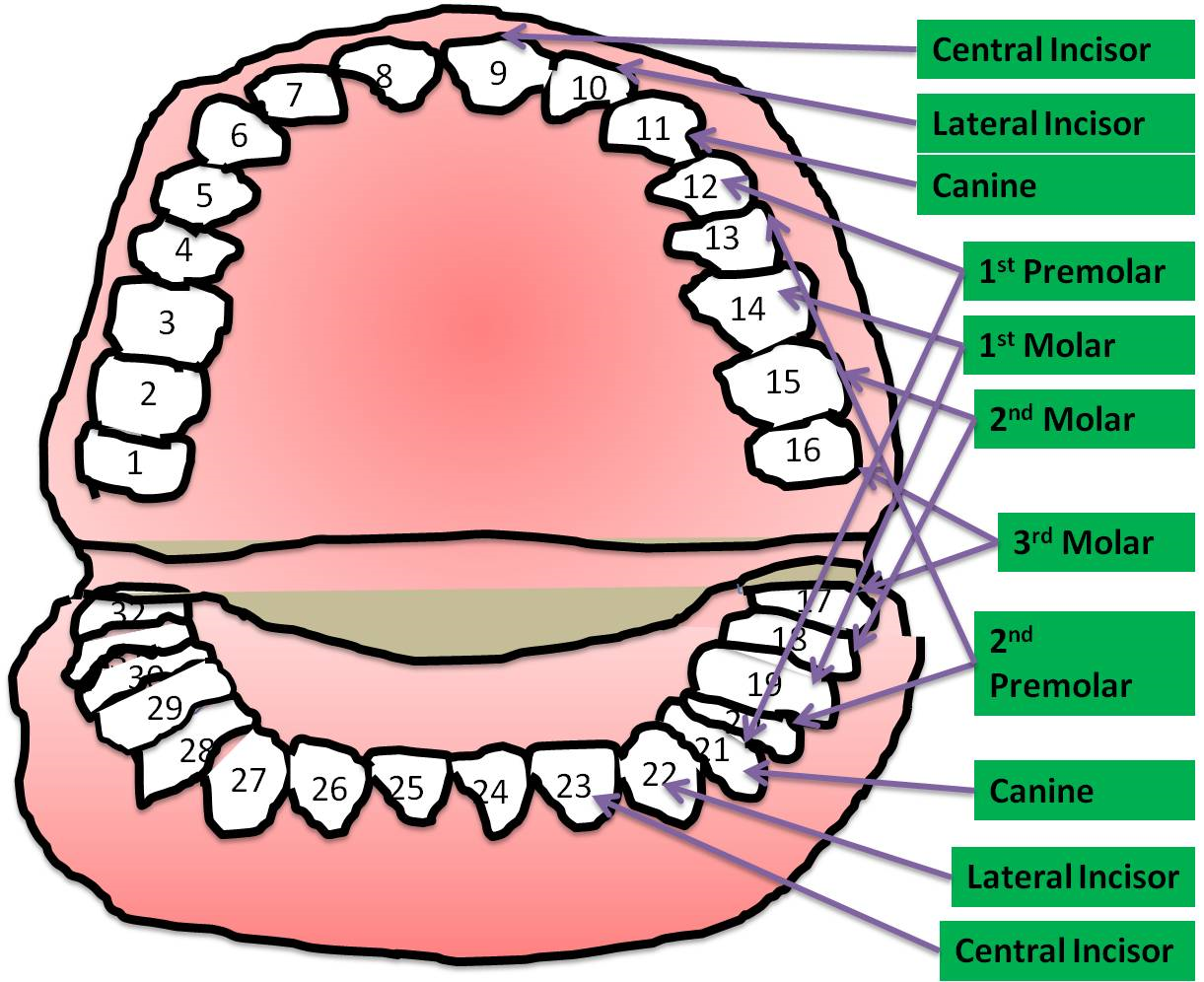
Figure 1. Adult human as 32 teeth having distinct names for them. As much as their position is situated in the corner of mouth where it is difficult to reach for brushing, the risk for developing infections also increases simultaneously. Most infections occur Premolar and molar teeth.
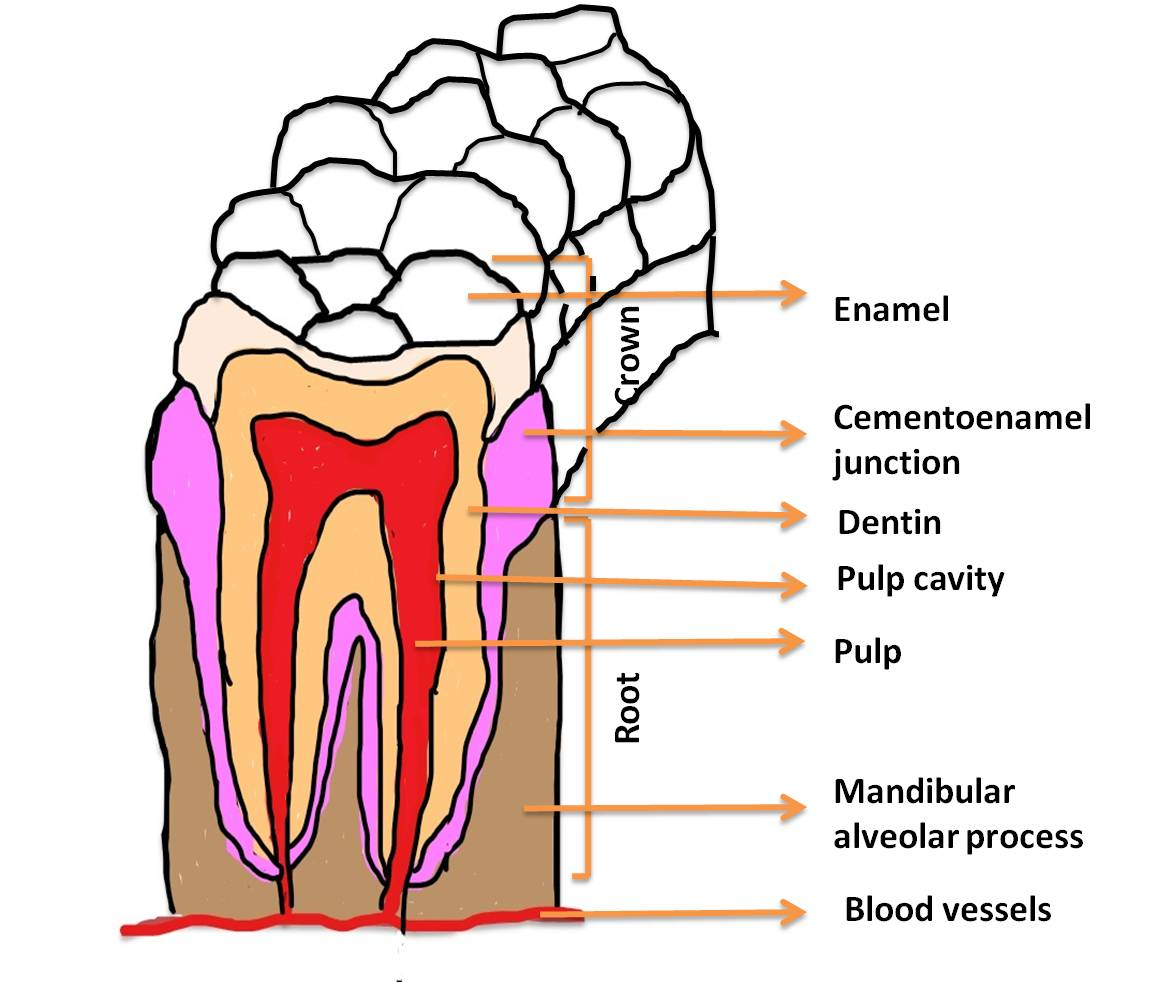
Figure 2. Different parts of a tooth has been indicated in the figure. To determine the disease conditions of a tooth, it is necessary to identify separate parts of a tooth because infection at these parts can be caused by separate pathogenic bacteria. The resulting infection in different portions might need distinct dental procedures to correct the situation.
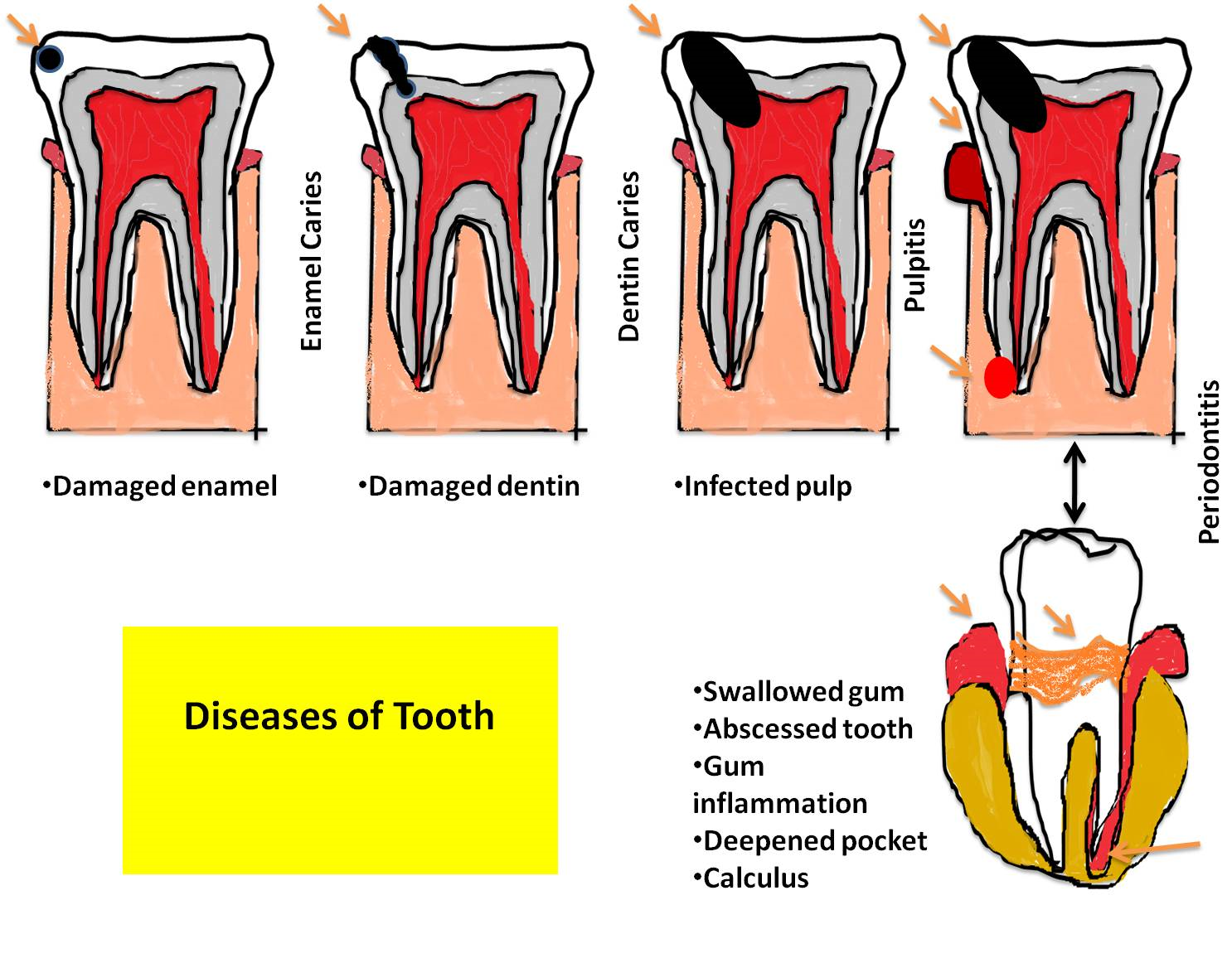
Figure 3. The dental caries is initiated by different ways. Different parts of the tooth can be affected during the progression of dental caries. As the condition becomes worse by further dissemination of the caries, the infection and disease symptoms increase. First the dental caries is initiated by the biofilm of pathogenic bacteria thriving in oral cavity if adequate cleaning is not regularly maintained. This initiation can be detected as a small caries on the enamel. If the condition is not corrected by proper treatment, the caries further progresses inside the tooth (dentin and then pulp). During this stage the microorganisms reach the pulp and abscess is formed followed by inflammation and deepened pocket calculus.
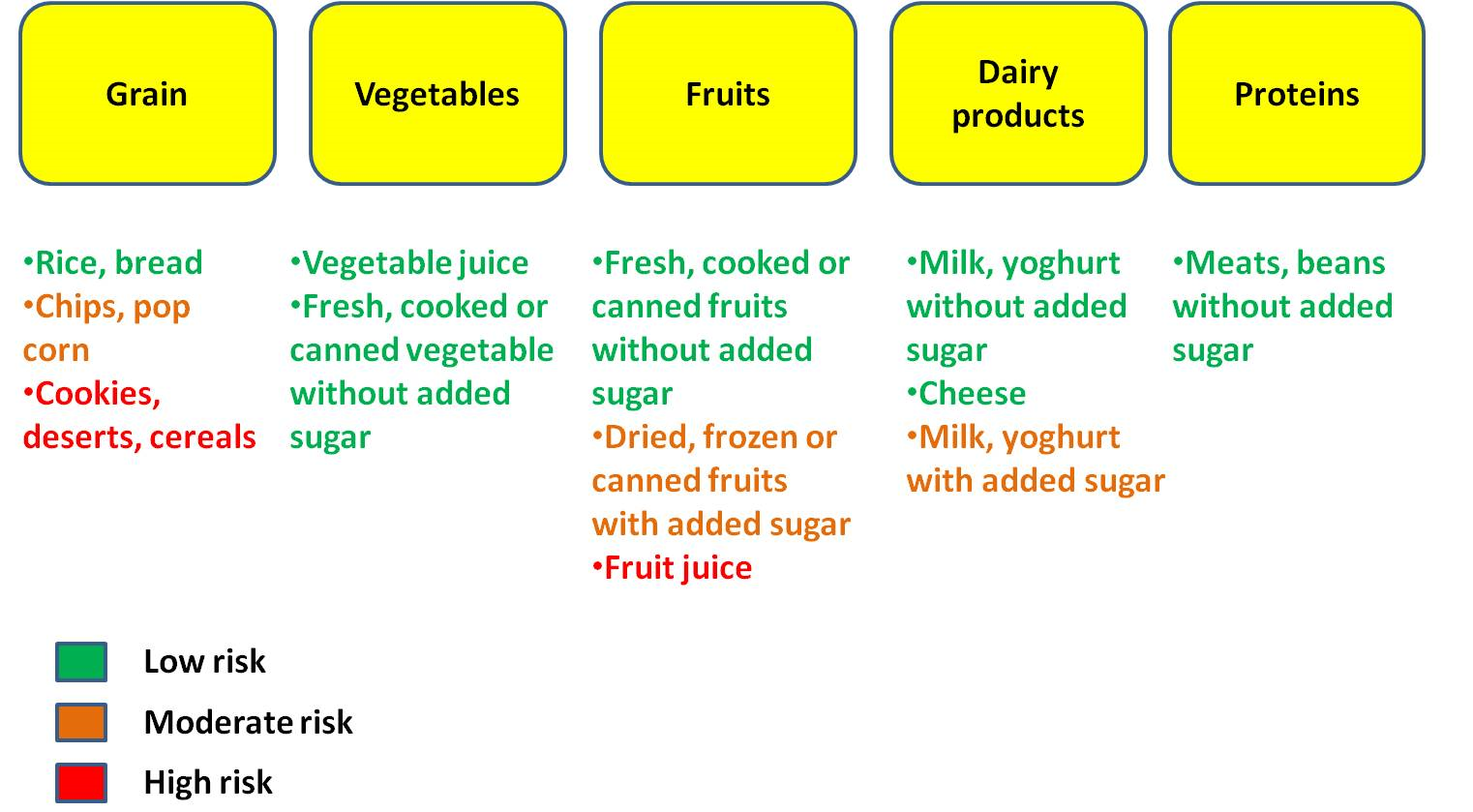
Figure 4. It is always better to take some preventive measures to decrease the chance of dental plaque formation. If the consumed food contains lots of sugar content it is very important to brush teeth as soon as possible to stop the accumulation of microbes on the tooth surface. Oral hygiene should be maintained strictly. Teeth should be brushed twice a day and after eating sweet food. Yearly dental check up is recommended to make early detection of caries and providing early treatment before making it worse.
Microbial biofilm formation is very common phenomenon in teeth which leads to the dental diseases starting with dental caries. Molar teeth (Figure 1) are most prone to such problems due to the difficulties in cleaning different food particles. Different parts of tooth are damaged during the progression of dental diseases (Figures 2, 3). After observing the disease symptoms as well as the microbiological analysis of the dental plaques, it has been revealed that certain types of microorganisms together forming dental plaques are responsible for certain symptoms (Table 1). Lactobacillus spp. and Streptococcus mutans were present in sample no. 1, 2, 3, 4 and in all these four cases there prevailed same types of symptoms including severe pain, restless night due to intensive pain. In all cases pain reduced for several hours and again started. Lactobacillus spp. and Streptococcus mutans together formed dental plaques in different tooth in these patients and maintaining oral hygiene was suggested. Root canal for the infected tooth was prescribed to remove the deep plaques and porcelain crown can be used depending on the condition of the tooth. Actinomycetes spp. was present in sample no. 7, 8 and 11. Capnocytophaga spp. (sample 7, 8), Treponema denticola and Porphyromonus gingivalis (sample 5,6,9) were also present. Control sample (healthy individual) was showed no growth of such bacteria.
Lactobacillus spp. is responsible for root caries in adults mostly. They can attach to the tooth surface easily and the most importantly they can co-aggregate with other species of microorganisms (especially Streptococcus spp.) and can initiate the biofilm production where prevails a complex interrelated metabolic reactions and functions (21). Streptococcus mutans in tooth surface utilizes sugar to produce lactic acid using the enzyme glucansucrase. The produced acid causes the mineralization of teeth enamel initiating the dental caries (22).
In patient no 5, there was pain at left 3,4,5,7, and right 2,6 teeth due to dental plaque formation and the microbiological analysis revealed the presence of Treponema denticola and Porphyromonus gingivalis. Dental scaling was suggested to remove the plaques and further maintenance of hygiene was advised. In patient no 5, there was also pain at left 1,2,3 and right 1,2,3 and the plaque forming microorganisms were Treponema denticola and Porphyromonus gingivalis like patient no 5. Treponema denticola is responsible for gum inflammation and periodontal disease. They can survive extreme harsh environment in mouth and make biofilms. In biofilms, they generally co-aggregate with Porphyromonus gingivalis (23,24). Porphyromonus gingivalis is one of the most common pathogen responsible for periodontic diseases. 85.75% samples of dental plaque showed the presence of this bacteria. It can survive in deep cavities where sugar availability is low and they can utilize amino acids instead of sugar. They can communicate with other bacteria increasing the advancement of the disease (25).
Patients 7 & 8 both showed similar symptoms like bad smell, bleeding Gum, bleeding continued while eating and brushing. Gingival swelling was also observed. In both patients, responsible microorganisms forming dental plaque were Actinomycetes spp. and Capnocytophaga spp. Capnocytophaga strains are isolated from dental plaques with other bacterial periodontal species of bacteria. This condition is responsible for alveolar bone loss, attachment loss, tooth mobility and tooth loss (26). Actinomycetes spp. can produce black stain or tooth discoloration, prone to calcification. They can also cause dental abscess (Figure 3).
Several studies were carried out before where similar microorganisms were identified causing distinct periodontal and gum disease (27-30).
Treatments include dental filling using amalgam which is silver-gray colored material. For better visualization, tooth colored filling components are also available. If the caries is big enough to be filled properly, artificial crown or cap is used as a cover over the damaged tooth. When the infection reaches the deepest part of tooth infecting the nerve n pulp, root canal is performed to remove the infected portion and filled with sealing material. And then a crown is placed over it, if necessary.
Dental caries is a very common dental problem occurring in the children as well as in adults. Many different types of microorganisms are responsible for dental caries. Streptococcus mutans, Lactobacillus spp., Actinomycetes spp., Capnocytophaga spp., Porphyromonas spp. etc bacteria have been identified from the patients subjected to microbiological analysis in this study. They can co-aggregate with each other enabling themselves to become more virulent and increase the disease symptoms and dental damages as well. Scaling, root cannel, fill ups and finally surgical removal of damaged tooth are the main treatments for the dental problems. Oral hygiene maintenance can decrease such problems.
Authors are thankful to the researchers of the related studies which have been cited in the text.
The authors reported no conflict of interest.
Received: 2019/10/16 | Accepted: 2020/01/19 | ePublished: 2020/01/19
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








